ORIGINAL
Protective Effect of Indomethacin-loaded Polymeric Nanoparticles Against Oxidative Stress-Induced Cytotoxicity in Human Breast Adenocarcinoma Cell Model
Efeito Protetor de Nanopartículas Poliméricas com Indometacina contra Citotoxicidade Induzida por Estresse Oxidativo em Modelo Celular de Adenocarcinoma de Mama Humano
Efecto Protector de Nanopartículas Poliméricas con Indometacina contra la Citotoxicidad Inducida por Estrés Oxidativo en Modelo de Células de Adenocarcinoma de Mama Humano
doi: https://doi.org/10.32635/2176-9745.RBC.2022v68n4.2545
Camila Franco1; Altevir Rossato Viana2; Aline Ferreira Ourique3; Bruno Stefanello Vizzotto4; Luciana Maria Fontanari Krause5
1-5Universidade Franciscana (UFN). Santa Maria (RS), Brazil.
1E-mail: cf@ufn.edu.br. Orcid iD: https://orcid.org/0000-0002-8793-6092
2E-mail: rossato.viana@hotmail.com. Orcid iD: https://orcid.org/0000-0002-0571-4219
3E-mail: aline.ourique@ufn.edu.br. Orcid iD: https://orcid.org/0000-0003-0828-774X
4E-mail: bvizzotto@ufn.edu.br. Orcid iD: https://orcid.org/0000-0001-6819-4081
5E-mail: lfontanari@yahoo.com.br. Orcid iD: https://orcid.org/0000-0001-8294-2533
Corresponding author: Camila Franco. Universidade Franciscana. Rua dos Andradas 1614 – Santa Maria (RS), Brazil. CEP 97010-032. E-mail: cf@ufn.edu.br
Abstract
Introduction: Anti-inflammatory drugs are being utilized to treat cancer because of its inflammatory microenvironment. Objective: The objective of this study is to investigate the antioxidant potential of indomethacin and its genotoxicity, since free or loaded in polymeric nanocapsules using MCF-7 (human breast cancer) cells as an in vitro model. Method: Development of indomethacin-loaded polyepsilon-caprolactone (PCL) nanocapsules by interfacial deposition method. It is characterized by pH determination by potentiometer, mean diameter and polydispersity index by dynamic light scattering; zeta potential by electrophoretic mobility; encapsulation efficacy by high performance liquid chromatography method; corona effect formation; 2ʹ,7ʹ-dichlorofluorescin diacetate (DCFH-DA) method by spectrofluorimetric assay; nitric oxide (NO) determination by spectrophotometric and genotoxicity assay by plasmid DNA cleavage method. Results: The results showed a mild acidic pH (4.78 ± 0.10), sizes around 200 nm and PDI<0.2 with a zeta potential around -20 mV and encapsulation efficiency of 99% (1 mg mL-1), showing a dose-dependent corona formation profile in 24h incubation. Conclusion: DCFH-DA assay showed no production of reactive oxygen species (ROS) while NO determination showed that Ind-OH-NC from 26.7 to 100 µM increased reactive nitrogen species (RNS), demonstrating antioxidant potential against MCF-7 cells. No sample at the concentrations evaluated induced DNA cleavage, being considered a safe treatment.
Key words: indomethacin/pharmacology; antioxidants; nanocapsules; neoplasms.
RESUMO
Introdução: Anti-inflamatórios estão sendo empregados para tratamento de câncer por causa do seu ambiente inflamado. Objetivo: Investigar o potencial antioxidante da indometacina e sua genotoxicidade, livre ou carreada em nanocápsulas poliméricas, usando como modelo in vitro células MCF-7 (câncer de mama humano). Método: Desenvolvimento de nanocápsulas de poliepsilon-caprolactona (PCL) por método de deposição interfacial, caracterizada por determinação de pH por potenciômetro; diâmetro médio e índice de polidispersão por espalhamento dinâmico de luz; potencial zeta por mobilidade eletroforética; eficiência de encapsulação por cromatografia líquida de alta eficiência; formação de efeito corona; método de 2’,7’-diclorofluoresceína diacetato (DCFH-DA) por ensaio espectrofluorimétrico; determinação de óxido nítrico (NO) por espectrometria e ensaio de genotoxicidade por método de clivagem do DNA plasmidial. Resultados: Os resultados mostraram leve pH ácido (4,78 ± 0,10), tamanhos em torno de 200 nm e PDI<0,2 com potencial zeta em torno de -20 mV e eficiência de encapsulação de 99% (1 mg mL-1), apresentando perfil de formação de corona dose-dependente em 24 horas de incubação. Conclusão: O ensaio DCFH-DA mostrou que não há produção de espécies reativas de oxigênio (ROS), enquanto a determinação de NO mostrou que Ind-OH-NC de 26,7 a 100 µM aumentou as espécies reativas de nitrogênio (RNS), demonstrando potencial antioxidante contra MCF-7. Nenhuma amostra nas concentrações avaliadas induziu clivagem do DNA, sendo considerado um tratamento seguro.
Palavras-chave: indometacina/farmacologia; antioxidantes; nanocápsulas; neoplasias.
RESUMEN
Introducción: Se están utilizando antiinflamatorios para tratamiento de cáncer debido a su entorno inflamado. Objetivo: Investigar el potencial antioxidante de la indometacina y su genotoxicidad, libre o acarreada en nanocápsulas poliméricas utilizando como modelo in vitro células MCF-7 (cáncer de mama humano). Método: Desarrollo de nanocápsulas de poli epsilon-caprolactona (PCL) por método de deposición interfacial, caracterizada por determinación de pH por potenciómetro; diámetro medio e índice de polidispersión por esparcimiento dinámico de luz; potencial zeta por movilidad electroforética; eficiencia de encapsulación por cromatografía líquida de alta eficiencia; formación de efecto corona; método de 2’,7’-diclorofluoresceína diacetato (DCFH-DA) por ensayo espectrofluorímetro; determinación de óxido nítrico (NO) por espectrometría y ensayo de genotoxicidad por método de clivaje del ADN plasmídico. Resultados: Los resultados mostraron ligero pH ácido (4,78 ± 0,10), tamaños alrededor de 200 nm y PDI<0,2 con potencial zeta alrededor de -20 mV y eficiencia de encapsulación de 99% (1 mg mL-1), presentando perfil de formación de corona dosis-dependiente en 24h de incubación. Conclusión: El ensayo DCFDA mostró que no hay producción de especies reactivas de oxígeno (ROS) mientras que la determinación de NO mostró que Ind-OH-NC de 26,7 a 100 µM aumentó las especies reactivas de nitrógeno (RNS), demostrando potencial antioxidante contra MCF-7. Ninguna muestra en las concentraciones evaluadas indujo clivaje del ADN, siendo considerado un tratamiento seguro.
Palabras clave: indometacina/farmacología; antioxidantes; nanocápsulas; neoplasias.
INTRODUCTION
Antineoplastic drugs have been traditionally used for the therapy of cancer, the most invasive disease of the world. Drugs from other therapeutic classes as the non-steroidal anti-inflammatory drugs (NSAID) are also employed in cancer treatment because cancerous regions are characterized by an inflammatory microenvironment1,2.
Inflammatory conditions are strictly associated with cancer development and tumor progression. It is known that chronic inflammation promotes carcinogenesis (proliferation, angiogenesis and metastasis) and reduces the immune response, as well as the action of chemotherapy drugs. Tumoral and inflammatory cells are similar while releasing cytokines and express inflammation receptors and mediators that induce DNA damage and tissues proliferation. It is already evidenced by a clinical assay that NSAID can interfere in the tumoral microenvironment reducing cell migration, increasing apoptosis and chemical sensibility3.
In addition to the inflammatory condition of cancerous regions, the oxidation damage contributes to the tumorigenicity, development, progression and recurrence. Free radicals are species with one or more unpaired electrons produced by metabolism (mainly in the mitochondria) or by external sources as ultraviolet radiation, exposition of chemical compounds, or exercise, which can react and contribute to the development of several diseases as aging-related processes, cardiovascular diseases, diabetes, renal ischemia, neurodegenerative disorders, immune system dysfunctions, inflammatory disease, hypoxia and cancers4,5.
Free radicals are classified into oxygen (ROS), nitrogen (RNS), sulfur (RSS) and chloride (RCS) reactive species. Of these, ROS are the most abundantly produced, including superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (OH-), singlet oxygen (1O2) and ozone (O3)5,6. RNS is basically nitric oxide (NO-) which can react with ROS and converted to hydroxyl radical or nitrite anion (NO2-) and are produced by enzymes existing in the mammary epithelial cells, dermic fibroblasts and keratinocytes5.
Since there is an alteration in the balance of antioxidant enzymes in the body and the production of ROS, these species can contribute to homeostasis maintenance when they are present at low concentrations, but at high concentrations, they can assume a pro-tumorigenic and a cytotoxic role. In this case, cancer cells develop an adaptative response to the microenvironment, accelerating their metabolism, and also produce ROS that affect neighboring cells and cause DNA damage (mutations), enhancing aging, angiogenesis and carcinogenesis5,7. It is known that the mitochondrial DNA is more easily oxidizable than the nuclear DNA4,6,8. In addition, ROS change proteins, leading to loss of function and causing lipid peroxidation reactions, increasing membrane permeability and leading to cell death5. On the other hand, RNS production at low levels is pro-angiogenic and pro-tumoral, leading to cell death by apoptosis and necrosis, while at high levels is considered as antineoplastic, being protective to the occurrence7,9,10 of apoptosis.
ROS intermediates (including nitric oxide and peroxynitrite) and contribute to a high expression of cyclooxygenase-2 enzyme (COX-2), which is the enzyme responsible for the prostaglandin synthesis, is involved in vascular tonus regulation and renal hydric balance11,12. NSAID are anti-inflammatory drugs that can inhibit COX-2 and consequently the inflammation process, and it has been demonstrated that they can act in the tumoral control by dependent mechanisms that have not yet been elucidated11,12.
The literature lists a variety of molecules with different associated therapeutic actions such as the anti-inflammatory, antineoplastic and antioxidant potentials. Among NSAID, there are some molecules with this multi-therapeutic action as aspirin and indomethacin, which have demonstrated to inhibit tumor proliferation of colon, gastric and esophageal cancers (aspirin) and HCA-7 cells (colon cancer, indomethacin)11-13. There are some evidences that naproxen, ibuprofen, sulindac and diclofenac have also shown antineoplastic action13,14.
Indomethacin is commonly used in clinic as an anti-inflammatory, antipyretic and analgesic drug, but causes an adverse effect as gastric mucosal injury, since it reduces the levels of a protein called survivin which is expressed on the surface of epithelial cells, providing the maintenance of its integrity. Indomethacin has already demonstrated antineoplastic potential for MDA MB-435 cells2, C6 glioma cells15, MCF-7 cells16 and once in polymeric nanoparticles, it could reduce their adverse effects17,18. Indomethacin also demonstrated antimicrobial, cytotoxic (by brine shrimp lethality test) and antioxidant potentials (by 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPF) method)19.
The use of anti-inflammatory compounds with associated antineoplastic and antioxidant activity may reduce the incidence and severity of the oxidative damage caused by ROS/RNS and contribute to tumoral suppression. Therefore, the aim of this study is to investigate the antioxidant potential of indomethacin and its genotoxicity since free or transported into polymeric nanocapsules using MCF-7 (human breast cancer) cells as model.
METHOD
PCL (Mw 14,000 g mol-1, Mn 10,000 g mol-1, Sigma Aldrich), polysorbate 80 (Tween 80Ò, Alpha Química), capric/caprylic triglyceride (CCT, Embacaps), indomethacin (99% purity, Sigma Aldrich), sorbitan monostearate, trypan blue (Sigma Aldrich), Dulbecco’s modified eagle medium (DMEM, Sigma Aldrich), penicillin and streptomycin (Merck), fetal bovine serum from Gibco (Thermo Fischer Scientific, Massachusetts, USA), 2ʹ,7ʹ-dichlorofluorescin diacetate (Sigma Aldrich, F7378), sodium nitrite (99%, Sigma Aldrich), sulfanilamide (99%, Sigma Aldrich), N-1-naphthylenediamine-dichlorohydrate (98%, Sigma Aldrich), pCMUT plasmid extracted from Escherichia coli strain DH10B, using Plasmid DNA Maxiprep kit (ThermoFisher Scientific), hydrogen peroxide, ascorbic acid, ferric chloride, Tris, boric acid, ethylenediamine tetraacetic acid (EDTA), GelRed (Sigma Aldrich) and TBE 1X (0.13 M tris (pH 7.6), 45 mM boric acid, 2.5 mM EDTA, Phoneutria).
High performance liquid chromatography method
A liquid chromatography (HPLC) method has been adapted20 and described16 for quantification of indomethacin.
Sample preparation
A solution of indomethacin was prepared at 26.7 µM (IC50 value on MCF-7 cell, tested from 1 to 75 µM by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in 24h, data not shown) and Ind-OH-NC method at 1 mg mL-1 was prepared21, and the formulation was described16.
Characterization of the nanocapsules formulation
The characterization of the nanocapsules formulation was performed as described16, analyzing the pH with a potentiometer (DM-22, Digimed Analytical Instrumentation, Brazil), previously calibrated with standard solutions, and the results were expressed as mean ± SD of the triplicate.
The drug extraction from the formulations was performed16 to obtain the total drug concentration, and the concentration of the drug in the continuous phase was obtained by the ultrafiltration-centrifugation method at 5000 rpm for 10 minutes (ultrafiltration units, Millipore®, 10 kDa, Irland; Microcentrifuge NT805, Brazil, n=3, obtaining the free drug concentration). The encapsulation efficiency (EE%) was calculated by dividing the difference of drug content and drug concentration in the continuous phase by the drug content, and multiplying by 100.
The z-average diameter (mean hydrodynamic diameter) and polydispersity index (relative variance, PDI) measurements were determined by the dynamic light scattering (DLS), in a Malvern Zetasizer instrument (NanoZS, ZEN 3600 model, Malvern Instruments, UK, 25oC, backscatter detection at 173o). Each sample (20 mL), without prior treatment, was diluted in 10 mL of ultrapure water (0.45 mm, MilliporeÒ, dilution of 500 times, n=3).
The zeta potential was determined by laser Doppler electrophoresis (DLS, NanoZS, ZEN 3600 model, Malvern Instruments, UK, 25oC), in which each sample (20 mL) was diluted in 10 mmol L-1 sodium chloride aqueous solution (10 mL), and placed in the folded capillary cell for analysis (n=3).
Detection of reactive oxygen species
The amount of total free radical was measured using the adapted22 2ʹ,7ʹ-dichlorofluorescein diacetate (DCFH-DA) assay.
The MCF-7 cell line (ATCC® HTB-22) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose level, supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin and maintained in a 5% CO2/95% air atmosphere at 37ºC, seeded in 96-well plates (2.104 cells per well) 24h before the application of the samples.
The samples evaluated were culture medium and hydrogen peroxide at 100 µM used as negative and positive control, respectively23; indomethacin solution at 26.7 µM and Ind-OH-NC at 1 mg mL-1 that was applied at 5, 26.7, 50 and 100 µM, diluted in the culture medium to a final volume of 125 µL/well. The assay was performed for 24, 48 and 72h of exposure to the treatments. After this time period, in a dark plate, 65 µL of 10mM Tris/HCl (tris(hydroxymethyl) aminomethane) buffer pH 7.4; 50 µL of the supernatant and 40 µL of 1mM DCFH-DA previously diluted 1:10 in ethanol were added and incubated for 30 minutes at room temperature and protected from light exposure (in the dark). Next, the fluorescent area was read with a spectrofluorometer (Synergy HTX reader, BioTek®, Winooski, USA) at 488/525 nm.
Nitric oxide test was also performed to evaluate the interference of the samples (culture medium, indomethacin solution at 26.7 µM and Ind-OH-NC at 1 mg mL-1 applied at 5, 26.7, 50 and 100 µM) on NO production. A curve with sodium nitrite was performed from 5 to 100 µM.
In a 96-well plate, 100 µL of the cell culture supernatant was pipetted, 100 μL of Griess reagent (previously prepared with 0.23 g of sulfanilamide, 0.012 g of N-(1-naphtyl) ethylenediamine dihydrochloride and 10 mL of phosphoric acid under steering and heat at 40ºC), left at room temperature for 15 minutes and read at 540 nm in the spectrophotometer (Synergy HTX reader, BioTek®, Winooski, USA)24.
Evaluation of the Corona effect
The Ind-OH-NC 1 mg mL-1 formulation was diluted in culture medium (DMEM) at concentrations of 5 and 100 µM, without addition of cells. These samples were incubated at 5% CO2/95% air atmosphere at 37°C, and analyzed in 24h, using as a parameter for comparison the results of characterization of the nanocapsules formulation characterization at day zero (day of production). These samples were evaluated by the dynamic light scattering technique using Zetasizer equipment (Zetasizer® nano-ZS model ZEN 3600, Malvern) for the characterization of the mean diameter, polydispersity index and zeta potential with a 500-fold dilution, besides its pH determination (DM-22, Digimed®, previously calibrated with standard solutions). All readings were performed in triplicate and the results expressed as mean ± standard deviation.
Genotoxicity
The ability of Ind-OH and Ind-OH NC to damage DNA molecule was evaluated after conversion of the supercoiled (FI) form of plasmid DNA, to the relaxed open circular form (FII). Concentrations of 10, 26.7 and 100 µM of the compounds were incubated with 300 ng of pCMUT plasmid for 1h at room temperature in triplicate. Then the formation of single-strand break DNA (SSBs) were quantitatively analyzed. Fenton reagent (30 mM H2O2, 50 mM ascorbic acid and 80 mM FeCl3) was used as the positive control (PC) and the pCMUT plasmid alone as the negative control (NC) of the test. To determine the average number of DNA lesions generated by the compounds, the relative amounts of FI and FII forms were measured after migration electrophoresis on 0.8% (w/v) agarose gel in TBE buffer (44.5 mM Tris pH 8.4, 44.5 mM boric acid, and 1 mM EDTA) at 100V for 2h at room temperature. The gels were stained with GelRed (SigmaAldrich®) and loading buffer solution (50 mM Tris-HCl at pH 7.5, 0.01% bromophenol blue, 50% glycerol and 250 mM EDTA) and the images were captured using the L-Pix Touch transilluminator (Loccus®, Cotia, SP-Brazil) under UV illumination. The fluorescence intensities of each DNA band and the relation between them were measured using the open-source software ImageJ® (v1.51)23.
Statistical analysis
All results were evaluated by one-way analysis of variance (ANOVA, 1 factor), followed by Tukey’s test, considering p-values <0.05 as significant. Graph Pad Prism version 5 was used to analyze cells culture results.
RESULTS
HPLC method
The HPLC calibration curve of indomethacin was validated25 in the range 1 to 30 µg mL-1, with correlation coefficient of 0.999 (y = 48190x-17287; retention time = 4.5 minutes, LD = 0.299 µg mL-1 and LQ = 0.997 µg mL-1). The method was linear, specific, reproducible and accurate (p = 0.011, Fcalc. = 4.55 and Fcrit. = 3.00).
Characterization of nanocapsules
The pH measurements of the nanocapsule formulations showed a slightly acidic 4.78 ± 0.10 for Ind-OH-NC. The drug content was 1 mg mL-1 (DC = 1.09 ± 0.12 mg mL-1), encapsulation efficiency of 99.04% and drug loading (%DL) of 2.11 ± 0.24. The formulations showed a mean Z diameter of 197.46 ± 2.05 nm, PDI of 0.134 ± 0.02 and zeta potential of -18.7 ± 0.85 mV.
Detection of reactive oxygen species
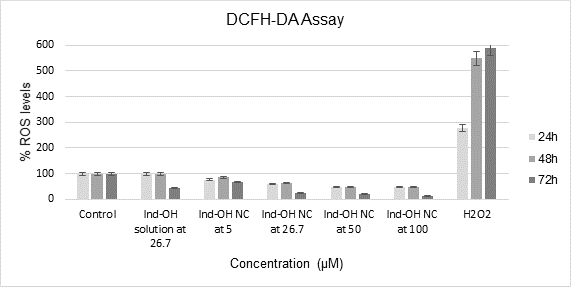
In the DCFH-DA assay, the results were collected as mean ± standard deviation (SD) of an n=8 replicate, and it was possible to observe that there was no ROS formation in 24, 48 or 72h as shown in Chart 1.
|
|
|
Chart 1. Ind-OH solution and Ind-OH NC evaluated by DCFH-DA assay in MCF-7 cells in 24, 48 and 72h of exposure, using culture medium and hydrogen peroxide as negative and positive control, respectively, the statistical differences between the samples were considered from p<0.05 and described in the text. ANOVA and Tukey test/24h: F = 121.98; Fcritic = 2.43; p = 7.02.10-24; HSD = 4330.2; ANOVA and Tukey test/48h: F = 134.35; Fcritic = 2.43; p = 1.05.10-24; HSD = 3660.7 and ANOVA and Tukey/72h: F = 20.83; Fcritic = 2.66; p = 1.13.10-7; HSD = 3970.36)
|
In Chart 1, it can be seen that, as expected, the positive control (H2O2 at 100 µM) shows a significant increase in DCF production, in relation to the negative control (DMEM). At 24h, all samples evaluated were different from the culture medium and Ind-OH solution at 26.7 µM was different from the Ind-OH NC from 5 to 100 µM. At 48h, all samples except the Ind-OH solution at 26.7 µM were different from the culture medium and the Ind-OH solution at 26.7 µM was different from Ind-OH NC from 5 to 100 µM. And after 72h of exposure, all samples were different from the culture medium but there were no differences between Ind-OH solution at 26.7 µM and the Ind-OH NC 26.7 µM. In addition to these differences, no sample presented an increase higher than 100% in ROS levels, therefore not influencing the production of ROS species.
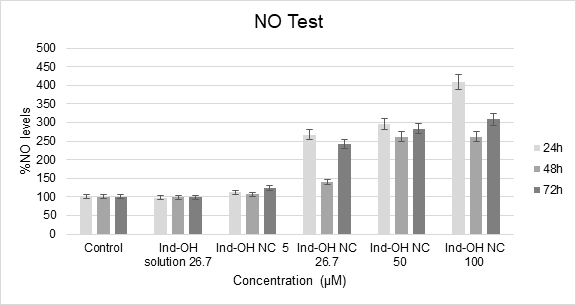
In the nitric oxide test, it was possible to observe that the samples Ind-OH NC from 26.7 to 100 µM were able to stimulate the NO production from MCF-7 cells, showing statistical difference compared to the control (DMEM), as presented in Chart 2.
|
|
|
Chart 2. Ind-OH solution and Ind-OH NC evaluated by NO test in MCF-7 cells in 24, 48 and 72h of exposure, using culture medium as the negative control. The statistical differences between the samples were considered from p<0.05 and described in the text. ANOVA and Tukey test/24h: F = 28.91; Fcritic = 2.43; p = 1.38.10-12; HSD = 0.106; ANOVA and Tukey test/48h: F = 53.01; Fcritic = 2.43; p = 4.59.10-17; HSD = 0.084 and ANOVA and Tukey/72h: F = 55.55; Fcritic = 2.43; p = 1.98.10-17; HSD = 0.080)
|
In 24h, it is possible to observe that Ind-OH-NC at 26.7 µM showed the RNS increase (corresponding to 81.12 µM of nitrite) due to a beginning fast drug delivery from the nanocarrier, but it was less pronounced in 48h, which had no statistical difference from the control due to the sustained release of indomethacin from its nanocarrier (maintenance dose). Also, it is known that nanoparticles may induce hormetic-like biphasic dose responses in a variety of cell types, displaying a stimulatory response in low doses (0-100 µg/mL, where Ind-OH-NC at 26.7µM correspond to 9.55 µg/mL) followed by a falling (0-50 µg/mL, where Ind-OH-NC at 50 µM correspond to 17.58 µg/mL and Ind-OH-NC at 100 µM correspond to 35.77 µg/mL) not exceeding 72h26. At 72h, Ind-OH-NC at 26.7 µM recovered the RNS production, probably due to cell adaptation to the environment and the accumulation of indomethacin doses released in a time-dependence27.
The same profile of RNS production occurred with Ind-OH-NC at 50 µM (corresponding to 92.86 µM of nitrite at 24h) and Ind-OH-NC at 100 µM. Ind-OH-NC at 100 µM showed a significant increase in RNS levels at 24h (corresponding to 140.85 µM of nitrite), which is dose-dependence followed by less pronounced increase of RNS levels in 48h and, a higher increase at 72h, justified by the indomethacin sustained release and accumulation in the culture medium.
Thus, it can be concluded that indomethacin does not influence ROS production, and Ind-OH-NC at 26.7 µM or at higher concentrations has antioxidant potential since it increases RNS levels.
Evaluation of the Corona effect
This assay demonstrated that the initial pH of the nanocapsule formulations diluted in DMEM medium was 7.94 ± 0.02 (day zero) and after 24h of incubation, it assumed the pH of 7.59 ± 0.01. Ind-OH-NC formulations presented an average Z-diameter of 272.47 ± 4.45 nm, a PDI of 0.25 ± 0.01 and zeta potential of -19.87 ± 1.27 mV without the dilution in DMEM. After 24h diluted in DMEM medium and incubated at 37ºC, Ind-OH-NC at 5 µM presented 241.97 ± 26.86 nm, PDI of 0.35 ± 0.04 and -9.54 ± 2.49 mV of zeta potential, while Ind-OH-NC at 100 µM had 498.17 ± 8.06 nm, PDI of 0.53 ± 0.05 and potential of -8.64 ± 2.89 mV. These results showed an increase in nanoparticle size around 216 nm, which is dose-dependent, and once nanoparticles with small sizes are considered at higher concentration, there is a higher surface area per volume, exposed to more reactions, so the nanoparticles could interact with the environment, do protein adsorption and thus corona formation.
The characterization made it possible to observe that the PDI increases, indicating the absence of particle size uniformity. The zeta potential was reduced due to the fact that amino acids or proteins in the environment can be interacting with the polymer (PCL) by their positive charge. Carboxylate-terminated molecules in biological media are deprotonated, generating a negative charge, as PCL and can adsorb the so-called soft corona (which is easily modified with protein changes, as it has a soft adsorption).
Genotoxicity
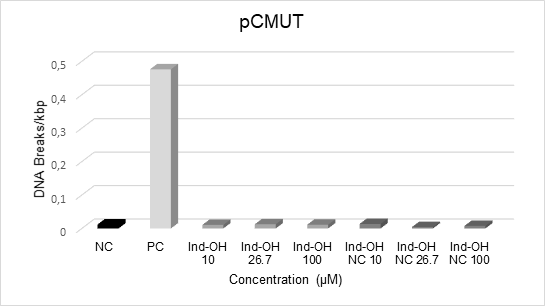
Considering that little is known about the safety of indomethacin-loaded nanoparticles, the induction of DNA damage by Ind-OH and Ind-OH-NC have been detected as changes in the plasmid forms, where the native plasmid (supercoiled) is transformed into the open circular form, mainly due to induced breaks in the DNA phosphate backbone, resulting in the formation of single strand breaks (SSBs). These breaks can also occur at both strands, resulting in the formation of double-strand breaks (DSBs), which can be seen as a strain on the gel image. The results of DNA damage quantification are depicted at Chart 3.
|
|
|
Chart 3. Induction of DNA damage by Ind-OH and Ind-OH-NC compounds at different concentrations. NC: negative control (plasmidial DNA only). PC: positive control (Fenton´s reagent). FI: plasmid DNA supercoiled form. FII: plasmid DNA relaxed form |
As observed, PC induced migration of FI plasmid to the FII form, mainly due to oxidative (hydroxyl radical) DNA lesions confirming the capacity of the assay to detect SSBs, while NC ensured the quality of pCMUT plasmid used in the assay. The results show that none of the compounds induced significant changes during the incubation period in the conformation of the DNA plasmid at all concentrations analyzed.
discussion
The HPLC method allowed the adequate quantification of indomethacin, showing that the formulation had a high encapsulation efficiency already described28. Concerning other physicochemical parameters, pH measurements demonstrated biocompatibility for the application of the formulation on skin (pH 4.0-7.0, depending on the region and type: dry or oily skin), once the extracellular pH of tumoral environment is acidic (around pH 5.0-7.0), this slightly acid pH does not attack the region and also is favorable to drug delivery from the nanocapsules29. PDI showed homogeneity of particle z-average diameter and zeta potential showed the stability of the formulation.
The DCFH-DA is deacetylated by esterase enzymes22, giving rise to 2‘,7’-dichlorodihydrofluorescein (DCFH, not fluorescent), which reacts with reactive oxygen species (ROS) and produces 2’,7’-dichlorofluorescein (DCF) that emits fluorescence. As already evidenced30, the MCF-7 cells when exposed to H2O2 at 25 and 250 µM during 24h, presented low levels of ROS that dose-dependently inhibit cell growth, acting as cytotoxic and apoptotic inducers. While after chronic exposure to H2O2 (of 3 months) there was an ROS accumulation that increased cell growth and survival, enhancing tumorigenicity and metastasis of breast cancer cells. There has been no study evaluating indomethacin free or within a nanocarrier by DCFH-DA assay to this moment.
The nitric oxide test is capable of detecting the presence of organic nitrite in the sample. Nitrite is detected and analyzed by the formation of a pink color when the Griess reagent is added to the NO2-containing sample due to the formation of diazonium salts that interact with the azo compound (N-1-naphthylenediamine-dichlorohydrate) and generate the pink color in the sample. Nitric oxide is a free radical with an extremely short life in biological systems, where endogenous production by nitric oxide synthase is established as playing an important role in vascular homeostasis, neurotransmission and host defense mechanisms31. The increase in the NO production may promote the reduction in signaling processes and oxidative stress that leads to cell death by apoptosis32. It has also been demonstrated that apoptosis occurs by caspase-dependent pathway or by the presence of other proteases or, also, by the Jun N-terminal kinase (JNK) pathway33. RNS formation is also associated with lipid peroxidation and membrane damage34. Furthermore, it is evidenced that NSAID can promote a cell cycle arrest in breast, lung, colon cancers and leukemia cancers35. Thus, it has been observed that indomethacin does not produce ROS or RNS and when encapsulated into nanoparticles its current antioxidant potential reduces signaling processes and leads to death by apoptosis, constituting an antineoplastic agent32.
The corona effect is the effect of biomolecules adsorption on the nanomaterials surface when exposed to biological microenvironments, including proteins, phospholipids, sugars, nucleic acids and others. This effect occurs due to the high-energy surface of the nanoparticles. The corona protein provides steric stabilization or destabilization by charge imbalance, altering the nanoparticles surface area, size, charge and its interaction with cell receptors, changing the cellular uptake mechanism and its bioavailability36,37. In addition, these particles can interact with cells in the microenvironment making changes, including damage such as oxidative stress and cytotoxicity37.
It has been evidenced that the positive corona does not cause cell damage, while negative ones can cause damage by ROS production using the corona from silica nanoparticles as a model37. This evidence concurs with the results found, since the negative zeta potential encountered in Ind-OH-NC particles is slightly negative and does not produce ROS in high levels, therefore it contributes to homeostasis.
The protein adsorption on the nanoparticle surface depends on their hydrophobicity and charge38. It is more important to consider the zeta potential in serum because the presence of corona effect is more relevant to promote (or not) the interaction with cells for an uptake and its therapeutic effect when transporting drugs than bare nanocapsules37. The corona protein is customized considering the environment and the pathology that affects the target tissue37.
Positively charged nanoparticles are easily internalized in cells by endocytosis, while negatively charged particles, such as Ind-OH-NC, could have affinity for cationic sites on the cell surface39. Once inside the cells, nanoparticles are enveloped by endosomes, being degraded by lysosomes or escaping from them and developing their therapeutic action or even being redirected to the extracellular environment.
Finally, the genotoxic effects of indomethacin-loaded Eudragit nanocapsules were evaluated by comet40 which is another important in vitro method to evaluate double and single-stranded genomic DNA breaks, showing that none of the tested formulations resulted in significant DNA damage in hepatoma cell line (HepG2 cells) and human peripheral blood lymphocytes. And when the interaction of indomethacin was analyzed with calf thymus DNA (Ct-DNA)41 through various biophysical techniques and in silico molecular docking, and even showing that the drug alone can form a complex with the minor groove of Ct-DNA, it was not able to induce the formation of SSBs or DSBs, proving the safety of indomethacin-based compounds at DNA level.
There is no report in the literature concerning the antioxidant potential of indomethacin nanoparticles applied to cancer treatment and its safety as well. The behavior of the formulation upon different pH’s (acid, neutral and basic, by Log D) to confirm the facilitated drug release in the acidic pH and its permeation and retention effect was not explored, which is a possible future exploration test that can be executed. Considering clinical applicability, there are studies with indomethacin nanoparticles carried in gel ointments to treat rheumatoid arthritis induced in rats42, and it could be evaluated in animals with tumor cells implants. The anti-inflammatories (free or nanocarried) must be better explored for its multi-therapeutic action and mechanisms of tumoral control.
CONCLUSION
Free indomethacin is not able to produce ROS or RNS in MCF-7 cells culture and once inside a polymeric nanocapsule from 26.7 µM to 100 µM increases the RNS species, which are considered antineoplastic. When RNS species are in the range (µM-mM), it induces apoptosis and nitrosative stress, inhibiting DNA synthesis and repair, suppressing the cellular respiration, enhancing inflammatory reactions, inhibiting metastasis, dilating tumor vessels and improving drug delivery. It was demonstrated that Ind-OH-NC promotes corona formation in dose-dependent manner when incubated with culture medium, which was confirmed by the reduction in zeta potential. Free indomethacin or Ind-OH-NC proved to be not-genotoxic and safe for administration.
ACKNOWLEDGEMENTS
To Universidade Franciscana for providing materials and infrastructure during the development of the experiments and to the investigator André Passaglia Schuch (Universidade Federal de Santa Maria) who kindly provided pCMUT to the group.
CONTRIBUTIONS
All the authors contributed to the study design/conception, acquisition, analysis and interpretation of the data, wording and critical review. They approved the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
FUNDING SOURCES
Scholarship of Scientific Initiation (PROBIC) provided by Universidade Franciscana.
REFERENCES
1. Rahme E, Ghosn J, Dasgupta K, et al. Association between frequent use of nonsteroidal anti-inflammatory drugs and breast cancer. BMC Cancer. 2005;5:159. doi: https://doi.org/10.1186/1471-2407-5-159
2. Ackerstaff E, Gimi B, Artemov D, et al. Anti-inflammatory agent indomethacin reduces invasion and alters metabolism in a human breast cancer cell line. Neoplasia. 2007;9(3):222-35. doi: https://doi.org/10.1593/neo.06673
3. Zappavigna S, Cossu AM, Grimaldi A, et al. Anti-inflammatory drugs as anticancer agents. In J Mol Sci. 2020;21(7):2605. doi: https://doi.org/10.3390/ijms21072605
4. Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231-55. doi: https://doi.org/10.1038/sj.bjp.0705776
5. Sosa V, Moliné T, Somoza R, et al. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376-90. doi: https://doi.org/10.1016/j.arr.2012.10.004
6. Crawford S. Anti-inflammatory/antioxidant use in long-term maintenance cancer therapy: a new therapeutic approach to disease progression and recurrence. Ther Adv Med Oncol. 2014;6(2):52-68. doi: https://doi.org/10.1177/1758834014521111
7. Oberley TD. Oxidative damage and cancer. Am J Pathol. 2002;160(2):403-8. doi: https://doi.org/10.1016/S0002-9440(10)64857-2
8. Kennedy RK, Veena V, Naik PR, et al. Phenazine-1-carboxamide (PCN) from pseudomonas sp. strain PUP6 selectively induced apoptosis in lung (A549) and breast (MDA MB-231) cancer cells by inhibition of antiapoptotic Bcl-2 family proteins. Apoptosis. 2015;20(6):858-68. doi: https://doi.org/10.1007/s10495-015-1118-0
9. Huerta S, Chilka S, Bonavida B. Nitric oxide donors: novel cancer therapeutics (review). Int J Oncol. 2008;33(5):909-27. doi: https://doi.org/10.3892/ijo_00000079
10. Galadari S, Rahman A, Pallichankandy S, et al. Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radic Biol Med. 2017;04:144-64. doi: https://doi.org/10.1016/j.freeradbiomed.2017.01.004
11. Chinery R, Beauchamp RD, Shyr Y, et al. Antioxidants reduce cyclooxygenase-2 expression, prostaglandin production, and proliferation in colorectal cancer cells. Cancer Res [Internet]. 1998 [cited 2021 Dec 14];58(11):2323-7. Available from: https://aacrjournals.org/cancerres/article-pdf/58/11/2323/2466382/cr0580112323.pdf
12. Kummer CL, Coelho TCRB. Antiinflamatórios não esteróides inibidores da ciclooxigenase-2 (COX-2): aspectos atuais. Rev Bras Anestesiol. 2002;52(4):498-512. doi: https://doi.org/10.1590/S0034-70942002000400014
13. Farrugia G, Balzan R. The proapoptotic effect of traditional and novel nonsteroidal anti-inflammatory drugs in mammalian and yeast cells. Oxid Med Cell Longev. 2013;2013:504230. doi: https://doi.org/10.1155/2013/504230
14. Pantziarka P, Sukhatme V, Bouche G, et al. Repurposing drugs in oncology (ReDO) – diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi: https://doi.org/10.3332/ecancer.2016.610
15. Bernardi A, Jacques-Silva MC, Delgado-Cañedo A, et al. Nonsteroidal anti-inflammatory drugs inhibit the growth of C6 and U138-MG glioma cell lines. Eur J Pharmacol. 2006;532(3):214-22. doi: https://doi.org/10.1016/j.ejphar.2006.01.008
16. Franco C, Silva ML, Viana AR, et al. Cytotoxicity evaluation of indomethacin-loaded polymeric nanoparticles in a human breast adenocarcinoma cell model. Braz J Dev. 2021;7(7):67004-14. doi: https://doi.org/10.34117/bjdv7n7-124
17. Yoshitomi T, Sha S, Vong LB, et al. Indomethacin-loaded redox nanoparticles improve oral bioavailability of indomethacin and suppress its small intestinal inflammation. Ther Deliv. 2014;5(1):29-38. doi: https://doi.org/10.4155/tde.13.133
18. Riasat R, Guangjun N, Riasat Z, et al. Effects of nanoparticles on gastrointestinal disorders and therapy. J Clin Toxicol. 2016;6(4):1000313. doi: https://doi.org/10.4172/2161-0495.1000313
19. Sukul A, Das SC, Saha JK, et al. Screening of analgesic, antimicrobial, cytotoxic and antioxidant activities of metal complexes of indomethacin. J Pharm Sci. 2014;13(2):175-80. doi: https://doi.org/10.3329/dujps.v13i2.21895
20. Pohlmann AR, Weiss V, Mertins O, et al. Spray-dried indomethacin-loaded polyester nanocapsules and nanospheres: development, stability evaluation and nanostructure models. Eur J Pharm Sci. 2002;16(4-5):305-12. doi: https://doi.org/10.1016/s0928-0987(02)00127-6
21. Bernardi A, Braganhol E, Jäger E, et al. Indomethacin-loaded nanocapsules treatment reduces in vivo glioblastoma growth in a rat glioma model. Cancer Lett. 2009;281(1):53-63. doi: https://doi.org/10.1016/j.canlet.2009.02.018
22. Esposti MD. Measuring mitochondrial reactive oxygen species. Methods. 2002;26(4):335-40. doi: https://doi.org/10.1016/S1046-2023(02)00039-7
23. Vizzotto BS, Dias RS, Iglesias BA, et al. DNA photocleavage and melanoma cells cytotoxicity induced by a meso-tetra-ruthenated porphyrin under visible light irradiation. J Photobiol B. 2020;209:111922. doi: https://doi.org/10.1016/j.jphotobiol.2020.111922
24. Choi WS, Shin PG, Lee JH, et al. The regulatory effect of veratric acid on NO production in LPS-stimulated RAW264.7 macrophage cells. Cell Immunol. 2012;280(2):164-70. doi: https://doi.org/10.1016/j.cellimm.2012.12.007
25. International Conference on Harmonization. Validation of analytical procedures: text and methodology Q2(R1) [Internet]. Current Step 4 version. Genebra: ICH; 2005 [cited 2021 Dec 14]. Available from: https://www.gmp-compliance.org/files/guidemgr/Q2(R1).pdf
26. Iavicoli I, Fontana L, Leso V, et al. Hormetic dose-responses in nanotechnology studies. Sci Total Environ. 2014;487:361-74. doi: https://doi.org/10.1016/j.scitotenv.2014.04.023
27. Remant-Bahadur KC, Thapa B, Xu P. pH and redox dual responsive nanoparticle for nuclear targeted drug delivery. Mol Pharm. 2012;9(9):2719-29. doi: https://doi.org/10.1021/mp300274g
28. Rota C, Chignell CF, Mason RP. Evidence for free radical formation during the oxidation of 2’-7’-dichlorofluorescin to the fluorescent dye 2’-7’-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Radic Biol Med. 1999;27(7-8):873-81. doi: https://doi.org/10.1016/s0891-5849(99)00137-9
29. Zhang X, Lin Y, Gillies RJ. Tumor pH and its measurement. J Nucl Med. 2010;51(8):1167-70. doi: https://doi.org/10.2967/jnumed.109.068981
30. Mahalingaiah PKS, Singh KP. Chronic oxidative stress increases growth and tumorigenic potential of MCF-7 breast cancer cells. PLoS ONE. 2014;9(1):e87371. doi: https://doi.org/10.1371/journal.pone.0087371
31. Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43(5):645-57. doi: https://doi.org/10.1016/j.freeradbiomed.2007.04.026
32. Sreenivasulua R, Reddya KT, Sujithab P, et al. Synthesis, antiproliferative and apoptosis induction potential activities of novel bis(indolyl)hydrazide-hydrazone derivatives. Bioorg Med Chem. 2019;27(6):1043-55. doi: https://doi.org/10.1016/j.bmc.2019.02.002
33. Tor YS, Yazan LS, Foo JB, et al. Induction of apoptosis in MCF-7 cells via oxidative stress generation, mitochondria-dependent and caspase-independent pathway by ethyl acetate extract of Dillenia suffruticosa and Its chemical profile. PLoS ONE. 2015;10(6):e0127441. doi: https://doi.org/10.1371/journal.pone.0127441
34. Szwed M, Torgersen ML, Kumari RV, et al. Biological response and cytotoxicity induced by lipid nanocapsules. J Nanobiotechnology. 2020;18(1):5. doi: https://doi.org/10.1186/s12951-019-0567-y
35. Park HB, Kim YJ, Lee SM, et al. Dual drug-loaded liposomes for synergistic efficacy in MCF-7 breast cancer cells and cancer stem cells. Biomed Sci Letters. 2019;25(2):159-69. doi: https://doi.org/10.15616/BSL.2019.25.2.159
36. Dror Y, Sorkin R, Brand G, et al. The effect of the serum corona on interactions between a single nano-object and a living cell. Sci Rep. 2017;7:45758. doi: https://doi.org/10.1038/srep45758
37. Westmeier D, Chen C, Stauber RH, et al. The bio-corona and its impact on nanomaterial toxicity. Eur J Nanomed. 2015;7(3):153-68. doi: https://doi.org/10.1515/ejnm-2015-0018
38. Barbosa KBF, Costa NMB, Alfenas RCG, et al. Estresse oxidativo: conceito, implicações e fatores modulatórios. Rev Nutr. 2010;23(4):629-43. doi: https://doi.org/10.1590/S1415-52732010000400013
39. Santos MC. Estudo do efeito do potencial de superfície na internalização de nanopartículas de magnetita em células cultivadas [trabalho de conclusão de curso na Internet]. Goiás: Universidade Federal de Goiás; 2007 [acesso 2021 dez 14]. Disponível em: https://www.prpg.ufg.br/up/85/o/modelo1.pdf
40. Froder JG, Dupeyrón D, Carvalho JCT, et al. In vitro study of the cytotoxic and genotoxic effects of indomethacin-loaded Eudragit(®) L 100 nanocapsules. Genet Mol Res. 2016;15(3). doi: https://doi.org/10.4238/gmr.15038727
41. Husain MA, Ishqi HM, Sarwar T, et al. Interaction of indomethacin with calf thymus DNA: a multi-spectroscopic, thermodynamic and molecular modelling approach. Medchemcomm. 2017;8(6):1283-96. doi: https://doi.org/10.1039/c7md00094d
42. Nagai N, Yoshioka C, Yoshimasa I. Topical therapies for rheumatoid arthritis by gel ointments containing indomethacin nanoparticles in adjuvant-induced arthritis rat. J Oleo Sci. 2015;64(3):337-46. doi: https://doi.org/10.5650/jos.ess14170
Recebido em 2/2/2022
Aprovado em 21/6/2022
Associate-Editor: Claudio Gustavo Stefanoff. Orcid iD: https://orcid.org/0000-0001-7050-3269
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer José Alencar Gomes da Silva | Ministério da Saúde