LITERATURE REVIEW
An Overview on the Evidence of Physical Activity Interventions in the Health of Individuals with Head and Neck Cancer: Literature Systematic Review
Um Panorama sobre as Evidências de Intervenções de Atividade Física na Saúde de Indivíduos com Câncer de Cabeça e Pescoço: Revisão Sistemática da Literatura
Un Panorama de las Evidencias de Intervenciones de Actividad Física en la Salud de Individuos con Cáncer de Cabeza y Cuello: Revisión Sistemática de la Literatura
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n1.2652
Patrícia Severo dos Santos Saraiva¹; Juliana da Silveira2; Jéssica Amaro Moratelli3; Kettlyn Hames Alexandre4; Mirella Dias5; Adriana Coutinho de Azevedo Guimarães6
1-4,6Universidade do Estado de Santa Catarina (Udesc). Florianópolis (SC), Brazil. E-mails: patricia.ed.fisica@hotmail.com; judasilveira88@gmail.com; jessica.moratelli@hotmail.com; kettlynhames@hotmail.com; adriana.guimaraes@udesc.br. Orcid iD: https://orcid.org/0000-0001-6122-9667; Orcid iD: https://orcid.org/0000-0003-2821-8717; Orcid iD: https://orcid.org/0000-0003-2007-4552; Orcid iD: https://orcid.org/0000-0002-6663-8926; Orcid iD: https://orcid.org/0000-0001-5167-2921
5Centro de Pesquisas Oncológicas (Cepon). Florianópolis (SC), Brazil. E-mail: mirelladias.fisio@gmail.com. Orcid iD: https://orcid.org/0000-0002-2109-3563
Corresponding author: Patrícia Severo dos Santos Saraiva. Udesc. Rua Pascoal Simone, 358 – Coqueiros. Florianópolis (SC), Brazil. 88080-350. E-mail: patricia.ed.fisica@hotmail.com
ABSTRACT
Introduction: Head and neck cancer is considered a global public health problem, which arises in aesthetically and functionally critical areas. The practice of physical exercise has been considered one of the significant and effective non-pharmacological strategies to minimize the physical and psychological consequences. Objective: To analyze the evidence of physical activity interventions in the physical and psychological health of individuals with head and neck cancer. Method: A systematic review was conducted blindly and independently, from March to May 2021, according to the PRISMA guidelines. The search was performed in the following databases: PubMed Central®; Cochrane Library; Web of Science, Scopus, ScienceDirect. Results: Of the 515 selected studies, 15 were included in this systematic review with a total of 670 participants aged between 18 and 76 years old. The studies included aerobic exercises, endurance, mobility, stretching, strengthening, and yoga. Conclusion: Evidence proves that physical activity interventions performed with individuals with head and neck cancer may be beneficial in the treatment and physical/psychological health of this population. This study may help new researches considering the detailed information described previously regarding the interventions applied, in addition to discussing the most used instruments with this public and indicating the modalities that are being safely performed. It is suggested that more randomized trials be conducted to obtain more concise results.
Key words: head and neck neoplasm/psychology; exercise therapy/psychology; exercise.
RESUMO
Introdução: O câncer de cabeça e pescoço é considerado um problema de saúde pública mundial, que surge em áreas cosmeticamente e funcionalmente críticas. A prática de exercício físico está sendo considerada uma das estratégias não farmacológicas significativas e eficazes a fim de minimizar as consequências físicas e psicológicas. Objetivo: Analisar as evidências de intervenções de atividade física na saúde física e psicológica de indivíduos com câncer de cabeça e pescoço. Método: Revisão sistemática de forma cega e independente, de março a maio de 2021, de acordo com as diretrizes PRISMA. A busca foi realizada nas seguintes bases de dados: PubMed Central®; Biblioteca Cochrane; Web of Science, Scopus, ScienceDirect. Resultados: Entre os 515 estudos selecionados, 15 foram incluídos nesta revisão sistemática com um total de 670 participantes com idade entre 18 e 76 anos. Os estudos incluíram exercícios aeróbicos, resistência, mobilidade, alongamento, fortalecimento e ioga. Conclusão: Evidências comprovam que intervenções de atividade física realizadas com indivíduos com câncer de cabeça e pescoço podem ser benéficas no tratamento e na saúde física/psicológica dessa população. Este estudo pode auxiliar em novas pesquisas considerando as informações detalhadas descritas anteriormente sobre as intervenções aplicadas, além de discutir os instrumentos mais utilizados com esse público e indicar as modalidades que estão sendo realizadas com segurança. Sugere-se a realização de mais ensaios randomizados para obter resultados mais concisos.
Palavras-chave: neoplasias de cabeça e pescoço/psicologia; terapia por exercício/psicologia; exercício físico.
RESUMEN
Introducción: El cáncer de cabeza y cuello es considerado un problema de salud pública a nivel mundial que se presenta en áreas estética y funcionalmente críticas. La práctica de ejercicio físico ha sido considerada una de las estrategias no farmacológicas significativas y eficaces para minimizar las consecuencias físicas y psíquicas. Objetivo: Analizar la evidencia de intervenciones de actividad física sobre la salud física y psicológica de individuos con cáncer de cabeza y cuello. Método: Revisión sistemática ciega e independiente de marzo a mayo de 2021, según las guías PRISMA. La búsqueda se realizó en las siguientes bases de datos: PubMed Central®; Biblioteca Cochrane; Web of Science, Scopus, ScienceDirect. Resultados: Entre los 515 estudios seleccionados, 15 fueron incluidos en esta revisión sistemática con un total de 670 participantes con edades entre 18 y 76 años. Los estudios incluyeron ejercicio aeróbico, resistencia, movilidad, estiramiento, fortalecimiento y yoga. Conclusión: La evidencia demuestra que las intervenciones de actividad física realizadas con individuos con cáncer de cabeza y cuello pueden ser beneficiosas en el tratamiento y la salud física/psicológica de esta población. Este estudio puede ayudar a futuras investigaciones considerando la información detallada descrita anteriormente sobre las intervenciones aplicadas, además de discutir los instrumentos más utilizados con esta audiencia e indicar las modalidades que se están realizando de forma segura. Se sugieren más ensayos aleatorios para obtener resultados más concisos.
Palabras clave: neoplasias de cabeza y cuello/psicología; terapia por ejercicio/psicología; ejercicio físico.
INTRODUCTION
Head and neck cancer belongs to a group of malignant neoplasms of the upper aerodigestive tract and the face located in the regions of the oral cavity, paranasal sinuses, salivary glands, pharynx, larynx, and thyroid that appears in critical areas and may produce changes in highly visible and socially significant portions1, often aggressive due to location and treatment2. As a consequence, survivors face specific physical, psychosocial problems, and symptoms related to the disease and its treatment such as oral dysfunction, shoulder mobility, facial lymphedema in addition to swallowing and speech problems that seriously compromise the health-related quality-of-life3-5.
Guidelines recommend that cancer survivors practice regular physical activities, performing at least 150 to 300 minutes of moderate-intensity aerobic physical activity or at least 75 to 150 minutes of vigorous aerobic physical activity. Another option would be an equivalent combination of moderate and vigorous activity during the week for substantial health benefits6. As a complementary therapy, physical exercise is considered one of the significant non-pharmacological strategies in the treatment of the disease, acting directly in physical and psychological health7, contributing positively to the physiological function of the individual, associated with the improvement of quality-of-life4,5,8. Therefore, it was verified in the literature that one of the first studies that addressed interventions involving physical exercises and physical activity directed to patients with head and neck cancer was a randomized clinical trial by McNeely et al.9, who evaluated the effects of progressive resistance exercise training on shoulder dysfunction, followed by Lønbro et al.10 who investigated the effect of progressive resistance training, followed by Samuel et al.11 which sought to evaluate the effects of physical training on functional capacity and quality-of-life.
Since then, few intervention studies involving this population have been performed, although literature reveals the existence of benefits in the plans of early physical exercises, aside from being viable and safe interventions with these patients12-14. However, the effectiveness of physical activity interventions related to this population is still considered to be inadequately understood13. Thus, the objective of this systematic review was to analyze the evidence of physical activity interventions in the physical and psychological health of individuals with head and neck cancer.
METHOD
This systematic review follows the guidelines of the Preferred Reporting Items for Systematic Reviews – PRISMA15, it was registered in PROSPERO (International Prospective Register of Systematic Reviews)16 - CRD42021265414, and its guiding question formed by the PICOS acronym is: What is the evidence of physical activity and/or physical exercise and body practices interventions in the physical and psychological health of adult individuals during and after head and neck cancer treatment?
Electronic researches were performed using the descriptors referred to in Chart 1 on five databases: PubMed Central®; Cochrane Library; - main collection, Web of Science; Scopus and ScienceDirect. All titles and abstracts found in the electronic search were analyzed through the application Rayyan and manually by two reviewers blindly and independently, in the period from 03/23/2021 to 05/16/2021. Reference lists of all relevant articles were examined to identify other eligible studies. The terms 'physical activity' and 'exercise' were used as a search strategy, with the intention of expanding the location of the greatest possible number of studies.
|
Chart 1. Complete search strategy in electronic databases, 2021 |
||||||||||
|
The researchers members of the Leisure and Physical Activity Research Laboratory - LAPLAF/CNPq, performed the searches according to the eligibility criteria. The discrepancies were solved by a third author.
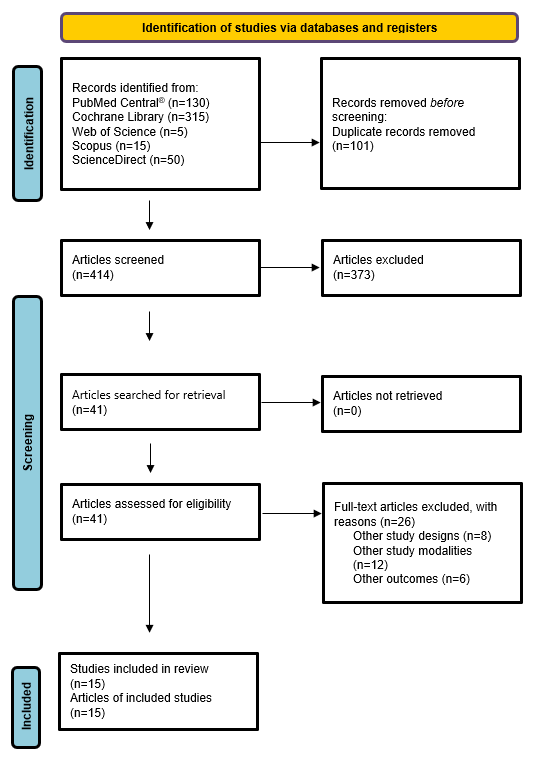
The eligibility criteria of the studies were defined according to the PICOS acronym considering the population, intervention, comparison, and study design (Chart 2). The eligible studies for this review were: a) randomized clinical trials; b) performed in adults (18 years); c) of both sexes; d) in treatment and post-treatment of head and neck cancer; e) published in English, Spanish and Portuguese in the last 10 years; f) studies that should investigate interventions with physical activities and/or physical exercise in the treatment of head and neck cancer, with summary and full text available during the period of 03/23/2021 to 05/16/2021. The information about the research is described by the reviewers in Figure 1, presented in the flowchart, with a description of the search, selection, inclusion, and exclusion process. The articles were initially classified and analyzed by title and the ones that did not meet the research criteria were excluded. The following action was reading abstracts, considering that articles in disagreement or duplicated were also removed. After the screening, the articles were read in full, so that the selection process of the studies would be completed.
|
Chart 2. Criteria for inclusion and exclusion of studies according to PICOS, 2021 |
||||||||||||||||||||||||
|
After searching studies for the systematic review, those addressing the effects of physical activity and/or physical exercise interventions on physical health outcomes (hemoglobin, platelet count, functional capacity, physical fitness, body composition, neck and shoulder function, nutritional status, shoulder range of motion, joint mobility of upper limbs and pain) and psychological health (quality-of-life, anxiety, depression, fatigue, fear, restlessness, nervousness, sleep disorders, and concentration) in individuals with head and neck cancer were included.
According to the World Health Organization (WHO)17, physical activity is defined as any body movement provided by skeletal muscles, thus generating energy expenditure. Regarding the complement to cancer therapies, physical exercise is clinically important to treatment, since it is accepted as a safe and effective method16. Therefore, at least 150 minutes of physical activity per week with moderate to vigorous intensity are recommended for cancer patients18.
Complex treatments for head and neck cancer have the potential to cause significant limitations, including pain, fatigue, and dysphagia19. Moreover, they also result in a decrease in the physical function of the upper limbs, a reduction in nutrition and communication, as well as dissatisfaction with body image and restriction of daily tasks20.
Head and neck cancer is considered one of the most psychologically traumatic cancers, due to the importance connected to the appearance of the head and neck21. Therefore, this type of cancer has the highest rates of major depressive disorders and it is associated with high levels of suffering22.
The investigation and discussion of the results were conducted through the extraction of data referring to the authors, year of publication, journal, the number of citations according to the Web of Science database (up to May 16, 2021), the number of databases, search period and followed up by the Checklist of 27 PRISMA items (yes or no). Next, it was obtained the objective, total sample size, age of the participants, intervention groups, control group, place of study, physical and psychological health investigated, characteristics of physical activity intervention and/or physical exercise, duration, number and frequency of sessions, intensity and duration of the intervention.
The Cochrane Collaboration tool23 was used to evaluate the methodological quality of the studies. The following criteria were evaluated: (1) generation of random sequence, (2) allocation sequence concealment, (3) masking of participants and researchers, (4) selective reports, (5) masking of results evaluation, and (6) incomplete results data. The researchers' understanding in this systematic review was evaluated as low, unclear, or high risk of bias.
RESULTS
There were 515 articles in the first search in the database, 130 in PubMed Central®, 315 in Cochrane Library, 5 in Web of Science, 15 in Scopus and 50 in ScienceDirect. After sorting the title and abstract, 490 articles were excluded because they did not meet the inclusion criteria, they were namely cross-references, were characterized by another type of study, animal studies, to investigate individuals with other diseases, under 18 years old, and without physical activity and/or physical exercise intervention. After completing the reading, 26 articles were excluded, totaling 15 articles4,10-12,14,24-33 included in this systematic review (Figure 1).
|
|
|
Figure 1. Flowchart of the study selection strategy, according to the PRISMA model, 2020 |
The study participants were all diagnosed with head and neck cancer in the laryngeal regions4,10,12,29,30, oropharynx4,12,28, tongue29,31,33, mouth10,12,27-30, retromolar trigone29, epiglottis29, amygdala29, pharynx10,12,27,30, nasopharynx33, thyroid25,31, parotid gland31, hypopharynx31,33, piriform sinus31, paranasal sinuses27 and salivary glands27. Five studies did not specify the region11,14,24,26,32.
The age of the participants of the studies varied according to the inclusion criterion: over 18 years10,27,28,30,33, from 18 to 75 years25, over 20 years old32, from 20 to 80 years29, from 32 to 76 years24 and six studies brought only the average age of the participants4,11,12,14,26,31.
The participants of the studies varied between men and women, being 494 men and 176 women, totaling 670 individuals, the study of Samuel et al.4 investigated the largest number of participants (n= 148 individuals) and Su et al.29, the smallest number (n= 37 individuals).
Regarding the stage of the disease, eleven studies had the characterization of their participants in the primary stage of cancer4,10,12,24,25,27-31,33 and the other four didn’t bring that information11,14,26,32.
Two studies brought participants who were submitted to the interventions receiving chemoradiotherapy4,11, three of them after head and neck cancer surgery14,29,31, one after radical neck dissection surgery26, three after radiotherapy10,28,33, one after chemotherapy32, during adjuvant treatment12, one after adjuvant treatment30, one with complete adjuvant therapy treatment24, one on medication with thyroid hormone after thyroidectomy25 and one after receiving primary radiotherapy and surgery with adjuvant treatment with or without chemotherapy27.
The year of publication of the studies was 201310,11, 201524,28, 201633, 201712,29, 201831, 20194,32 and 202014,26,27,30.
The studies were developed in Asia, in India4,11,26, Taiwan29,32, and South Korea25,31; in Europe, in Denmark10,12,28,30, in Germany14 and ending in North America in Canada24,27,33.
Among the studies, only seven were registered in clinical trials platforms, in the Clinical Trials10,12,28,30,33, German Clinical Trials14 and Government of India clinical trial records26.
It was observed that fourteen studies used control group, one study received, in the control group, recommendations to practice walking for 10 minutes, 5 days a week4; two received no intervention other than conventional care (cancer follow-up visits)30,32; one received standard clinical physiotherapy14, two received standard hospital care11,12, one received usual care offered by an oncologist and other health professionals as well as mouth opening exercises28, two received home exercise program29,31, two received 12 weeks of guidance only for self-directed activities, followed by 12 more weeks of intervention (resistance training)10,33, one received exercises of muscular energy techniques26, one received dynamic resistance exercises compatible with training volume27, one received usual care, with postural exercises and strengthening24 and finally a study provided no instruction25.
The interventions were: aerobic exercises (walking)4,11,25,32, endurance exercise4,11,12,32; physical therapies (including aerobic, anaerobic, and stretching therapies)29; yoga30; range of motion exercises, massage, stretching, and strengthening26,31; standard clinical physiotherapy exercises (mobilization movements)14; home exercise program with resistive training25,33; progressive endurance training10; exercises of stretching, postural and strengthening with maximum load and repetitions24; eccentric strength training with overload and neuromuscular electrical stimulation27; exercises for jaw mobility28.
The period of interventions ranged from 10 days26; 4 weeks31; 5 to 6 weeks (during the period of radiotherapy treatment)28; 6 weeks11; 8 weeks14; 11 weeks4; 12 weeks25,27,29,30,33; 24 weeks10; 12 weeks24 and a study did not specify12.
The frequency proposed for interventions varied once a week28, twice a week33; two and three times a week12; three times a week24,27,31,32; three to five days a week25,29; five days a week4,11; 30 sessions over 12 weeks10, 3rd and 5th post-operative day26 and two studies did not bring this information14,30.
The duration of each session of the speeches ranged from 60 minutes29; 40 to 50 minutes32; 40 minutes31; 30 to 40 minutes25; 45 minutes28 and ten studies did not specify the duration of the sessions4,10-12,14,24,26,27,30,33.
The intensity of the interventions was light to moderate4; moderate25,29,32,33; vigorous24 and nine studies did not report the intensity10-12,14,26-28,30,31.
The intensity was evaluated using the Borg Rating of Perceived Exertion Scale4,11,29,31, based on the American College of Sports Medicine, with the calculation of maximum frequency32 and seven studies did not specify12,14,26-28,30,33.
Among the professionals who performed the activities, there are physical therapists14,26,28,31; nutritionists30; clinical support with an exercise physiologist or personal trainer33; exercise specialist for cancer population27; support of physiotherapists and occupational therapists12; doctors and nurses11; academics and unsupervised professionals10; four studies were conducted at home and did not provide specifications on the professionals involved4,24,25,29 and a study brought no information32.
The interventions were performed in a hospital31; home4,12,25,29,33; home and rehabilitation center30; home and hospital28; physiotherapy department14; commercial training center facilities10; university24,27 and three studies failed to report11,26,32.
The outcomes evaluated were divided into physical and psychological health. All studies were proposed to evaluate participants in the baseline and post-intervention periods. Some studies included evaluations in the middle of the intervention, in the 3rd and 7th weeks4, in the 12th week33, on the 3rd and 5th day26, and in the 6th week29. Studies including follow-up evaluations were also found between 5 and 12 months after the intervention28, 36 and 48 weeks after the intervention33, 2, 5 and 12 months after the intervention12, 24 weeks10, 12 months after intervention24.
The physical outcomes evaluated in the clinical trial studies of this systematic review were functional capacity4,10-12,29,30,33, hemoglobin and platelet count4, range of motion29, pain12,26,29, body composition10,12,30,32,33, neck and shoulder function24,26,28,31, cardiovascular functions14,32, physiological responses32, respiratory function14, skeletal muscle functions14, digestive tract functions14, nutritional status12,33, neuromuscular function27, trismus12,28, aspiration and penetration12, xerostomia (dry mouth)12, muscle strength10, level of physical activity24 and immunological function25.
The psychological outcomes evaluated in the clinical trial studies of this systematic review were quality-of-life4,10,11,24,25,27,28,30,31,33, depression30,33, anxiety25,30, fear14, restlessness14, nervousness14, sleep disorders14 and concentration14.
Therefore, in this section, the characteristics of the studies included in this review, as shown in chart 3 and 4 will be described.
|
Chart 3. Details of the selected studies concerning the participants and the control group/comparison group, 2021 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
Chart 4. Details of selected studies about the characteristics of the interventions, physical and psychological health investigated, outcomes and instruments, 2021 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The studies presented a higher risk of bias about "masking participants and team" and "other sources of bias" and most of them were classified as high and uncertain because they did not offer sufficient data for the evaluation. Nevertheless, it should be noted that the category that presented a low risk of bias was a "random sequence generation" where it was considered low for almost all studies. This item corresponds to the method used to generate the allocation sequence of participants randomly.
DISCUSSION
The physical health variables investigated in the studies included in this review were hemoglobin, platelet count, functional capacity, physical fitness, body composition, neck and shoulder function, nutritional status, range of movement, joint mobility, and pain, however, the most prominent in the studies were functional capacity, neck and shoulder function, joint mobility and range of movement, which corroborate the findings of Lynch et al.13. Studies4,11,12,25,29,32,33 show that aerobic exercises such as walking combined with resisted exercises such as endurance training are presented in most of the studies since they have improved functional capacity, mobility, and cardiopulmonary capacity predominantly. Aerobic and endurance exercises alone improved muscle strength, physical fitness, nutritional status, shoulder range of movement, pain, and quality-of-life.
Exercises for jaw joint mobility also stood out in this review, since they relate to the needs associated with cancer location. Other interventions, as flexibility, stretching, and Yoga were also applied, but without significant results for this population.
Among the psychological health variables, it is highlighted the quality-of-life, depression, anxiety, and fatigue, but others were also investigated as fear, restlessness, nervousness, sleep disorders, and concentration. As well as in physical health, the interventions that stood out most in the improvement of these symptoms were also aerobic exercises and resistance combined, which showed a significant improvement in the quality-of-life, anxiety, and fatigue. Thus, the findings in the literature reinforce that combined exercises (aerobic and resistance exercises) are efficient in other types of cancers, such as breast cancer, which improved quality-of-life and reduced fatigue effects34, corroborating the data found in this review.
Yoga also improved the quality-of-life of head and neck cancer patients, similarly to other studies for individuals with breast cancer, such as Jong et al.35 and Dhruva et al.36, plus improvement in anxiety36, quality of sleep36,37 and reduction of fatigue38,39.
In this systematic review, the analysis of the study by Lavigne et al.27, indicated that the exercise intervention with the aid of neuromuscular electrical stimulation, besides the increase of the quality-of-life, brought benefits in fatigue reduction, as well as other studies which addressed the same intervention to individuals with head and neck cancer resulting in improved swallowing, prevention of dysphagia40, improvement of shoulder functions, in addition to pain reduction, proving effective for shoulder rehabilitation41 with significant improvements in quality-of-life42.
Most of the studies did not provide information about the time of the sessions and the intensity of the exercises, making it difficult to perceive their influence in the interventions and being a limitation for their replication. Bull et al.6 state in their research that, performing 150-300 minutes of activity with moderate to vigorous intensity with a frequency of 3 to 5 days a week brings benefits to the physical and psychological health of individuals with cancer, meeting the studies in this review, where those who brought a more significant improvement for their participants were those who had the highest frequency (from 3 to 5 days per week) of physical activity and/or physical exercise6.
In this sense, the practice of exercise offers benefits before, during, and after the treatment of cancer, in its various types and for various deficiencies related to it43, which meets the results of this review, since the participants were mostly in the process of treatment or after treatment for head and neck cancer.
Some interventions were home-based without direct guidance. The assisted interventions counted with the presence of physical therapists and exercise professionals with or without expertise in cancer patients. In addition to home, the interventions were applied at hospitals/clinics, and training centers. It cannot be stated whether or not this factor influenced the results, because some studies, even with the help of a professional, did not obtain the expected benefits. Moreover, this meets the findings of the study by Cantwell et al.44, which shows that there are some barriers health professionals have in promoting physical exercise programs for cancer patients, however, they play an important role in motivating individuals to adopt positive lifestyle changes.
Stout et al.43 show that exercise when supervised produces great benefits if compared to unsupervised. On the other hand, Su et al.29, Capozzi et al.33, Samuel et al.4, Hajdú et al.12, and Kim et al.25 studies applied home interventions, therefore, it can be implied that if the patient follows the appropriate guidelines, good results can be achieved. Considering that interventions with aerobic and resistance exercises are the most used for this population, with positive effects on quality-of-life and physical health in general which validates the findings of Lynch et al.13, that also revealed that cancer head and neck survivors adhered to these activities in their routines.
According to the National Cancer Institute (INCA)45 there is evidence that physical activity has a beneficial effect for people with cancer as it contributes to hormonal regulation, increased immunity and decrease oxidative stress from fat in addition to carcinogenesis46. There were also findings on the safety of physical activity for people with cancer, which were deemed as safe and well tolerated during and after treatment for various types of cancer46,47.
There was a consensus among the studies4,11,29,32,33 regarding the choice of the instrument for verification of physical health, wherein in a great part, the 6-minute Walk Test (6MWT) was used and considered an effective, low-cost and easy to apply tool in the evaluation of the functional capacity of healthy individuals or individuals with chronic diseases48. For psychological health, the instruments validated for individuals with head and neck cancer that stood out the most were the EORTC QLQ-C30, EORTC H&N 35, FACT H & N, and S-3610,25,27-31. These tools are also suitable for assessing the quality-of-life49, aiming to understand the true impact of head and neck cancer in this population, enabling a better therapeutic choice, thus assisting in rehabilitation and psychosocial support50.
As physical activity and/or exercise interventions cannot be blinded, as participants are aware of the type of intervention they are receiving as well as they need to understand the activity they are developing. Due to this, this item in the methodological quality of the studies had a high risk of bias, consequently, the study may be influenced by changes in the conduct of the research team or participants51.
The information on intensity in the studies analyzed was scarce or non-existent, as well as volume and frequency, which decreases the quality since intensity is a primary variable to define the effectiveness of an intervention and meets the results of a systematic review of exercises in the cancer literature (2005-2017), where moderate and vigorous-intensity exercises may be equally better compared to low-intensity exercise interventions43.
The reduced number of studies and protocols involving physical activity for the head and neck cancer population, as well as the lack of information on intensity, duration and frequencies of interventions, which may hinder the replicability of future interventions are some of the study limitations like the use of different instruments to verify the same variables which may bring unequal results.
CONCLUSION
It can be stated that physical activity interventions in individuals with head and neck cancer improve the symptoms evaluated in this population, but even so, there is a gap in the literature on that matter, especially those investigating psychological health. Therefore, it appears to be evident that further randomized controlled and high-quality studies need to be carried out to determine the type, intensity, frequency, and ideal moment of physical activity interventions, as well as its impact on cancer prognosis. It is also considered that few studies of physical interventions and HNC survivors were found, with a limited number of samples, making methodological evaluation difficult.
However, when considering the use of these interventions for clinical use, there is a gap in including therapeutic treatments in complementation of the traditional ones, corroborating the findings in this review, indicating that the practice of physical activity can contribute to the improvement of the health of cancer survivors potentially mitigating the likely side effects of pharmacological therapies and possibly achieving a better response to the treatment as a whole.
The present study is strong in its assertions as it may contribute to future investigations considering the information about interventions practice. Moreover, it suggests that psychological variables should be further studied due to possible complications caused by the disease. It is beneficial as well for health professionals and the scholar community making clear the relevance of physical exercise and physical activity. More studies are necessary on that matter.
CONTRIBUTIONS
Patrícia Severo dos Santos Saraiva and Juliana da Silveira contributed to the study design, acquisition, analysis and interpretation of the data, wording and critical review. Jéssica Amaro Moratelli, Kettlyn Hames Alexandre, Mirella Dias and Adriana Coutinho de Azevedo Guimarães contributed to the study wording and critical review. They approved the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
This study was funded in part by the Coordination for the Improvement of Higher Education Personnel (Capes) – Brazil. Finance Code 0001.
REFERENCES
1. Silva FA, Roussenq SC, Tavares MGS, et al. Perfil epidemiológico dos pacientes com câncer de cabeça e pescoço em um Centro Oncológico no Sul do Brasil. Rev Bras Cancerol. 2020;66(1):e-08455. doi: https://doi.org/10.32635/2176-9745.RBC.2020v66n1.455
2. Bye A, Sandmael JA, Stene GB, et al. Exercise and nutrition interventions in patients with head and neck cancer during curative treatment: a systematic review and meta-analysis. Nutrients. 2020;12(11):3233. doi: https://doi.org/10.3390/nu12113233
3. Nogueira TE, Adorno M, Mendonca E, et al. Factors associated with the quality-of-life of subjects with facial disfigurement due to surgical treatment of head and neck cancer. Med Oral Patol Oral Cir Bucal. 2018;23(2):e132-e7. doi: https://doi.org/10.4317/medoral.22072
4. Samuel SR, Maiya AG, Fernandes DJ, et al. Effectiveness of exercise-based rehabilitation on functional capacity and quality-of-life in head and neck cancer patients receiving chemo-radiotherapy. Support Care Cancer. 2019;27(10):3913–20. doi: https://doi.org/10.1007/s00520-019-04750-z
5. Senchak JJ, Fang CY, Bauman JR. Interventions to improve quality-of-life (QOL) and/or mood in patients with head and neck cancer (HNC): a review of the evidence. Cancers Head Neck. 2019;4:2. doi: https://doi.org/10.1186/s41199-019-0041-4
6. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451-62. doi: https://doi.org/10.1136/bjsports-2020-102955
7. Odynets T, Briskin Y, Todorova V. Effects of different exercise interventions on quality-of-life in breast cancer patients: a randomized controlled trial. Integr Cancer Ther. 2019;18:1534735419880598. doi: https://doi.org/10.1177/1534735419880598
8. Xiao C, Beitler JJ, Higgins KA, et al. Pilot study of combined aerobic and resistance exercise on fatigue for patients with head and neck cancer: inflammatory and epigenetic changes. Brain Behav Immun. 2020;88:184-92. doi: https://doi.org/10.1016/j.bbi.2020.04.044
9. McNeely ML, Parliament M, Courneya KS, et al. A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head Neck. 2004;26(6):518-30. doi: https://doi.org/10.1002/hed.20010
10. Lønbro S, Dalgas U, Primdahl H, et al. Progressive resistance training rebuilds lean body mass in head and neck cancer patients after radiotherapy – Results from the randomized DAHANCA 25B trial. Radiother Oncol. 2013;108(2):314-9. doi: https://doi.org/10.1016/j.radonc.2013.07.002
11. Samuel SR, Maiya GA, Babu AS, et al. Effect of exercise training on functional capacity & quality-of-life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res [Internet]. 2013 [cited 2021 May 15];137:515-20. Available from: https://journals.lww.com/ijmr/Fulltext/2013/37030/Effect_of_exercise_training_on_functional_capacity.11.aspx
12. Hajdú SF, Wessel I, Johansen C, et al. Swallowing therapy and progressive resistance training in head and neck cancer patients undergoing radiotherapy treatment: randomized control trial protocol and preliminary data. Acta Oncol. 2017;56(2):354-9. doi: https://doi.org/10.1080/0284186X.2016.1269193
13. Lynch PT, Horani S, Lee R, et al. Effectiveness of physical activity interventions in improving objective and patient-reported outcomes in head and neck cancer survivors: a systematic review. Oral Oncol. 2021;117:105253. doi: https://doi.org/10.1016/j.oraloncology.2021.105253
14. Steegmann J, Bartella AK, Kloss-Brandstätter A, et al. A randomized clinical trial on the efficacy of a patient-adapted autonomous exercise regime for patients with head and neck cancer. J Craniomaxillofac Surg. 2020;48(3):187-92. doi: https://doi.org/10.1016/j.jcms.2019.12.009
15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: https://doi.org/10.1136/bmj.n71
16. Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1:2. doi: https://doi.org/10.1186/2046-4053-1-2
17. World Health Organization [Internet]. Geneva: World Health Organization; c2022. Physical activity; 2022 Oct 5 [cited 2022 Aug 30]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/physical-activity
18. Spence RR, Sandler CX, Newton RU, et al. Physical activity and exercise guidelines for people with cancer: Why are they needed, who should use them, and when? Semin Oncol Nurs. 2020;36(5):151075. doi: https://doi.org/10.1016/j.soncn.2020.151075
19. Rodriguez AM, Komar A, Ringash J, et al. A scoping review of rehabilitation interventions for survivors of head and neck cancer. Disabil Rehabil. 2019;41(17):2093-2107. doi: https://doi.org/10.1080/09638288.2018.1459880
20. Swore Fletcher B, Cohen MZ, Schumacher K, et al. A blessing and a curse. Cancer Nurs. 2012;35(2):126-32. doi: https://doi.org/10.1097/NCC.0b013e31821bd054
21. Howren MB, Christensen AJ, Karnell LH, et al. Psychological factors associated with head and neck cancer treatment and survivorship: evidence and opportunities for behavioral medicine. J Consult Clin Psychol. 2013;81(2):299-317. doi: https://doi.org/10.1037/a0029940
22. Archer J, Hutchison I, Korszun A. Mood and malignancy: head and neck cancer and depression. J Oral Pathol Med. 2008;37(5):255-70. doi: https://doi.org/10.1111/j.1600-0714.2008.00635.x
23. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: https://doi.org/10.1136/bmj.d5928
24. McNeely ML, Parliament MB, Seikaly H, et al. Sustainability of outcomes after a randomized crossover trial of resistance exercise for shoulder dysfunction in survivors of head and neck cancer. Physiother Can. 2015;67(1):85-93. doi: https://doi.org/10.3138/ptc.2014-13O
25. Kim K, Gu MO, Jung JH, et al. Efficacy of a home-based exercise program after thyroidectomy for thyroid cancer patients. Thyroid. 2018;28(2):236-45. doi: https://doi.org/10.1089/thy.2017.0277
26. Thomas A, D’Silva C, Mohandas L, et al. Effect of Muscle Energy Techniques V/S active range of motion exercises on shoulder function post modified radical neck dissection in patients with head and neck cancer - A randomized clinical trial. Asian Pac J Cancer Prev. 2020;21(8):2389-93. doi: https://doi.org/10.31557/APJCP.2020.21.8.2389
27. Lavigne C, Twomey R, Lau H, et al. Feasibility of eccentric overloading and neuromuscular electrical stimulation to improve muscle strength and muscle mass after treatment for head and neck cancer. J Cancer Surviv. 2020;14(6):790-805. doi: https://doi.org/10.1007/s11764-020-00893-9
28. Høgdal N, Juhl C, Aadahl M, et al. Early preventive exercises versus usual care does not seem to reduce trismus in patients treated with radiotherapy for cancer in the oral cavity or oropharynx: a randomised clinical trial. Acta Oncol. 2015;54(1):80-7. doi: https://doi.org/10.3109/0284186X.2014.954677
29. Su TL, Chen AN, Leong CP, et al. The effect of home-based program and outpatient physical therapy in patients with head and neck cancer: a randomized, controlled trial. Oral Oncol. 2017;74:130-4. doi: https://doi.org/10.1016/j.oraloncology.2017.10.002
30. Kristensen MB, Wessel I, Beck AM, et al. Effects of a multidisciplinary residential nutritional rehabilitation program in head and neck cancer survivors-results from the NUTRI-HAB randomized controlled trial. Nutrients. 2020;12(17):2117. doi: https://doi.org/10.3390/nu12072117
31. Do JH, Yoon IJ, Cho YK, et al. Comparison of hospital based and home based exercise on quality-of-life, and neck and shoulder function in patients with spinal accessary nerve injury after head and neck cancer surgery. Oral Oncol. 2018;86:100-4. doi: https://doi.org/10.1016/j.oraloncology.2018.06.019
32. Yen CJ, Hung CH, Kao CL, et al. Multimodal exercise ameliorates exercise responses and body composition in head and neck cancer patients receiving chemotherapy. Support Care Cancer. 2019;27(12):4687-95. doi: https://doi.org/10.1007/s00520-019-04786-1
33. Capozzi LC, McNeely ML, Lau HY, et al. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: results from an exploratory randomized controlled exercise trial. Cancer. 2016;122(8):1185-200. doi: https://doi.org/10.1002/cncr.29863
34. Pagola I, Morales JS, Alejo LB, et al. Concurrent exercise interventions in breast cancer survivors with cancer-related fatigue. Int J Sports Med. 2020;41(11):790-7. doi: https://doi.org/10.1055/a-1147-1513
35. Jong MC, Boers I, Schouten van der Velden AP, et al. A randomized study of yoga for fatigue and quality-of-life in women with breast cancer undergoing (Neo) adjuvant chemotherapy. J Altern Complement Med. 2018;24(9-10):942-53. doi: https://doi.org/10.1089/acm.2018.0191
36. Dhruva A, Miaskowski C, Abrams D, et al. Yoga breathing for cancer chemotherapy-associated symptoms and quality-of-life: results of a pilot randomized controlled trial. J Altern Complement Med. 2012;18(5):473-9. doi: https://doi.org/10.1089/acm.2011.0555
37. Chaoul A, Milbury K, Spelman A, et al. Randomized trial of Tibetan yoga in patients with breast cancer undergoing chemotherapy. Cancer. 2018;124(1):36-45. doi: https://doi.org/10.1002/cncr.30938
38. Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32(10):1040-9. doi: https://doi.org/10.1200/JCO.2013.51.8860
39. Taso CJ, Lin HS, Lin WL, et al. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer. J Nurs Res. 2014;22(3):155-64. doi: https://doi.org/10.1097/jnr.0000000000000044
40. Bhatt AD, Goodwin N, Cash E, et al. Impact of transcutaneous neuromuscular electrical stimulation on dysphagia in patients with head and neck cancer treated with definitive chemoradiation. Head Neck. 2015;37(7):1051-6. doi: https://doi.org/10.1002/hed.23708
41. Baldwin ERL, Baldwin TD, Lancaster JS, et al. Neuromuscular electrical stimulation and exercise for reducing trapezius muscle dysfunction in survivors of head and neck cancer: a case-series report. Physiother Can. 2012;64(3):317-24. doi: https://doi.org/10.3138/ptc.2011-23O
42. Langmore SE, McCulloch TM, Krisciunas GP, et al. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: a randomized clinical trial. Head Neck. 2016;38(Suppl 1):E1221-31. doi: https://doi.org/10.1002/hed.24197
43. Stout NL, Baima J, Swisher AK, et al. A systematic review of exercise systematic reviews in the cancer literature (2005-2017). PM R. 2017;9(9S2):S347-84. doi: https://doi.org/10.1016/j.pmrj.2017.07.074
44. Cantwell M, Walsh D, Furlong B, et al. Healthcare professionals’ knowledge and practice of physical activity promotion in cancer care: challenges and solutions. Eur J Cancer Care (Engl). 2018;27(2):e12795. doi: https://doi.org/10.1111/ecc.12795
45. Instituto Nacional de Câncer José Alencar Gomes da Silva. Dieta, nutrição, atividade física e câncer: uma perspectiva global: um resumo do terceiro relatório de especialistas com uma perspectiva brasileira [Internet]. Rio de Janeiro: INCA; 2020 [acesso 2022 ago 30]. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/dieta_nutricao_atividade_fisica_e_cancer_resumo_do_terceiro_relatorio_de_especialistas_com_uma_perspectiva_brasileira.pdf
46. Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375-90. doi: https://doi.org/10.1249/MSS.0000000000002116
47. World Cancer Research Fund International; American Institute for Cancer Research. Physical activity and the risk of cancer [Internet]. London: WCRF; 2018 [cited 2022 Aug 30]. Available from: https://www.wcrf.org/wp-content/uploads/2021/02/Physical-activity.pdf
48. Falvo MJ, Earhart GM. Six-minute walk distance in persons with parkinson disease: a hierarchical regression model. Arch Phys Med Rehabil. 2009;90(6):1004-8. doi: https://doi.org/10.1016/j.apmr.2008.12.018
49. Wan Leung S, Lee TF, Chien CY, et al. Health-related quality-of-life in 640 head and neck cancer survivors after radiotherapy using EORTC QLQ-C30 and QLQ-H&N35 questionnaires. BMC Cancer. 2011;11:128. doi: https://doi.org/10.1186/1471-2407-11-128
50. Vartanian JG, Carvalho AL, Yueh B, et al. Brazilian-Portuguese validation of the University of Washington Quality-of-life Questionnaire for patients with head and neck cancer. Head Neck. 2006;28(12):1115-21. doi: https://doi.org/10.1002/hed.20464
51. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: Cochrane Collaboration; 2011 [cited 2022 Mar 11]. Available from: https://handbook-5-1.cochrane.org/whnjs.htm
Recebido em 14/4/2022
Aprovado em 13/9/2022
Associate-Editor: Fernando Lopes Tavares de Lima. Orcid iD: https://orcid.org/0000-0002-8618-7608
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde