Factors Associated with Karnofsky Performance Status and its Trajectory in the Last Month of Life in Patients with Terminal Cancer
Fatores Associados ao Karnofsky Performance Status e sua Trajetória no Último Mês de Vida em Pacientes com Câncer Terminal
Factores Asociados al Karnofsky Performance Status y su Trayectoria en el Último Mes de Vida en Pacientes Oncológicos Terminales
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n1.2754
Andrezza Helena Regadas Muniz1; Karla Santos da Costa Rosa2; Juliana Miranda Dutra de Resende3; Simone Garruth dos Santos Machado Sampaio4; Livia Costa de Oliveira5
1-5Instituto Nacional de Câncer (INCA), Unidade de Cuidados Paliativos. Rio de Janeiro (RJ), Brazil. E-mails: andrezzaregadas@hotmail.com; kcostarosa@gmail.com; julidutr@gmail.com; simonegarruth@gmail.com; lillycostaoliveira@gmail.com. Orcid iD: https://orcid.org/0000-0002-1619-9486; Orcid iD: https://orcid.org/0000-0002-0951-8725; Orcid iD: https://orcid.org/0000-0003-4232-4233; Orcid iD: https://orcid.org/0000-0001-5537-7399; Orcid iD: https://orcid.org/0000-0002-5052-1846
Corresponding author: Livia Costa de Oliveira. INCA. Unidade de Cuidados Paliativos. Rua Visconde de Santa Isabel, 274 – Vila Isabel. Rio de Janeiro (RJ), Brazil. CEP 20560-120. E-mail: lillycostaoliveira@gmail.com
ABSTRACT
Introduction: Karnofsky Performance Status (KPS) can characterize the impact of the disease on cancer patients. Objective: To evaluate the factors associated with KPS and its trajectory in the last month of life in patients with terminal cancer. Method: Retrospective cohort study, with terminal cancer patients enrolled in a Palliative Care Unit, who died between July and August 2019. The dependent variable was the KPS assessed daily in the last month of life. A cross-sectional analysis of factors associated with initial KPS was performed using ordinal logistic regressions. To verify the trajectory of KPS in the last month of life, longitudinal graphic analyzes were performed. Results: 108 patients were evaluated, most of whom were >60 years old (68.5%) and female (62.0%). The most prevalent tumor sites were the gastrointestinal tract (GIT) (24.3%), breast (18.7%) and head and neck (HN) (16.8%). In the multiple model, the primary tumor sites remained associated with KPS. During the last month of life, the reduction in KPS was more pronounced in those with tumors in the GIT, HN and connective bone tissue, who had higher KPS values on the thirtieth day before death when compared to the others. On the other hand, those with central nervous system and lung cancer started the follow-up period with lower KPS values and had a less exacerbated reduction than the others. Conclusion: KPS values decrease in the last month of life, but with different intensity according to the tumor site in patients with terminal cancer.
Key words: Karnofsky performance status; neoplasms; terminally Ill; palliative care; prognosis.
RESUMO
Introdução: O Karnofsky Performance Status (KPS) pode caracterizar o impacto da doença em pacientes com câncer. Objetivo: Avaliar os fatores associados ao KPS e a sua trajetória no último mês de vida em pacientes com câncer terminal. Método: Estudo de coorte retrospectivo, com pacientes com câncer terminal internados em uma unidade de cuidados paliativos, falecidos entre julho e agosto de 2019. A variável dependente foi o KPS avaliado diariamente no último mês de vida. Uma análise transversal dos fatores associados ao KPS inicial foi realizada por meio de regressões logísticas ordinais. Para verificar a trajetória do KPS no último mês de vida, foram realizadas análises gráficas longitudinais. Resultados: Foram avaliados 108 pacientes, cuja maioria possuía >60 anos (68,5%) e era do sexo feminino (62,0%). Os sítios tumorais mais prevalentes foram o trato gastrointestinal (TGI) (24,3%), mama (18,7%) e cabeça e pescoço (CP) (16,8%). No modelo múltiplo, os sítios tumorais primários permaneceram associados ao KPS. Durante o último mês de vida, a redução do KPS foi mais pronunciada naqueles com tumor no TGI, CP e tecido ósseo conjuntivo, que apresentaram valores mais elevados de KPS no trigésimo dia antes do óbito quando comparados aos demais. Por outro lado, aqueles com câncer no sistema nervoso central e pulmão iniciaram o período de seguimento com valores de KPS mais baixos e tiveram redução menos exacerbada que os demais. Conclusão: Os valores de KPS diminuem no último mês de vida, porém com intensidade diferente de acordo com o local do tumor em pacientes com câncer terminal.
Palavras-chave: avaliação de estado de Karnofsky; neoplasias; doente terminal; cuidados paliativos; prognóstico.
RESUMEN
Introducción: Karnofsky Performance Status (KPS) puede caracterizar el impacto de la enfermedad en pacientes con cáncer. Objetivo: Evaluar los factores asociados al KPS y su trayectoria en el último mes de vida en pacientes con cáncer terminal. Método: Estudio de cohortes retrospectivo, con pacientes oncológicos terminales ingresados en una Unidad de Cuidados Paliativos, fallecidos entre julio y agosto de 2019. La variable dependiente fue el KPS valorado diariamente en el último mes de vida. Se realizó un análisis transversal de los factores asociados con KPS inicial mediante regresiones logísticas ordinales. Para verificar la trayectoria de KPS en el último mes de vida, se realizaron análisis gráficos longitudinales. Resultados: Se evaluaron 108 pacientes, la mayoría con >60 años (68,5%) y del sexo femenino (62,0%). Los sitios tumorales más prevalentes fueron el tracto gastrointestinal (TGI) (24,3%), mama (18,7%) y cabeza y cuello (CC) (16,8%). En el modelo múltiple, los sitios del tumor primario permanecieron asociados con KPS. Durante el último mes de vida, la reducción de KPS fue más pronunciada en aquellos con tumores en TGI, CC y tejido conectivo óseo, quienes tenían valores de KPS más altos en el trigésimo día antes de la muerte en comparación con los demás. Por otro lado, aquellos con cáncer de sistema nervioso central y pulmón comenzaron el período de seguimiento con valores más bajos de KPS y tuvieron una reducción menos exacerbada que los demás. Conclusión: Los valores de KPS disminuyen en el último mes de vida, pero con distinta intensidad según la localización del tumor en pacientes con cáncer terminal.
Palabras clave: estado de ejecución de Karnofsky; neoplasias; enfermo terminal; cuidados paliativos; pronóstico.
INTRODUCTION
Functional capacity is often used to characterize the disease’s impact on cancer patients, it is not only an indicator of overall performance but also of the ability to perform activities of daily living. In addition, it is considered a valuable element for prognostic evaluation, and prevention of adverse effects associated with functional decline1.
For Sanvezzo et al.2 the loss of functionality is concomitant with disease progression, impairing the individual to perform basic activities and reducing his or her independence, negatively reflecting in the quality-of-life and survival. Likely, it occurs due to probable alterations in the cognitive, locomotor and communication systems, essential for daily tasks, either due to cancer treatment or to the advanced disease itself3.
It can be measured with scales evaluating the patients in their activities of daily life and need of regular medical care due to the onset of the disease, an important indicator of health and quality of life in palliative care, which consequently helps to direct clinical decision making1.
The Karnofsky Performance Status (KPS) is a tool used in clinical practice developed in 1949 by Karnofsky and Burchenal4 preferentially used for patients with terminal cancer in palliative care as a percentage scale. It is contingent upon several factors and is directly related to oncology prognosis indicating that as functionality worsens, survivorship decreases5.
Most cancer patients are able to keep their functionality for a substantial period of time. In more advanced phases of the disease, the KPS decreases and even plummets in the last weeks and days of life5. However, there is scarce evidence in the scientific literature to demonstrate the factors associated with KPS and the longitudinal behavior until death of patients with terminal cancer. Thus, the aim of this study is to assess KPS associated factors and its trajectory in the last month of life in patients with terminal cancer.
METHOD
The retrospective cohort study was carried out with terminal cancer patients who died during hospitalization, between June and August 2019, at INCA’s Palliative Care Unit (PCU). The eligibility criteria were: age ≥ 20; confirmed diagnosis of malignant tumor in terminal stage, regardless of location; date of death between June and August 2019 and admitted at the unit for more than 30 days before the study period. The Institutional Review Board of the National Cancer Institute (INCA) approved the study, report number 3,899,964, dated March 5, 2020. The signature of the Informed Consent Form was waived.
One skilled investigator collected the data from electronic charts. The KPS is an 11-point percentage scale ranging from 100% (normally active) to 0 (death) and is the dependent variable of the study obtained by chart review, daily, 30 days before death. The study’s baseline was the 30th day before death. Karnofsky and Burchenal4 routinely used for multidisciplinary assessment of all patients, in outpatient consultations, home-based or during hospitalization.
In addition, sociodemographic data as age, gender, skin color and clinical data such as the primary tumor site [upper and lower gastrointestinal tract (GIT) vs. breast vs. head and neck (HN) vs. gynecological vs. lung vs. connective bone tissue (CBT) vs. central nervous system (CNS) vs. others], disease progression, previous treatment, nutritional risk and the serum levels of albumin were evaluated.
Nutritional risk was evaluated using the Portuguese validated version of the Patient-Generated Subjective Global Assessment Short Form (PG-SGA SF) (©FD Ottery, 2005, 2006, 2015), available by Ottery at Pt-Global6. Patients with an overall score >9 points were classified as being at nutritional risk7.
Analyzes were performed using Stata Data Analysis and Statistical Software (STATA) version 13.0. Values were considered statistically significant when p-value <0.05.
The Kolmogorov Smirnov test was applied to assess the distribution of the data. Continuous numeric variables were described as mean and standard deviation or as median and interquartile range according to the sample distribution, while categorical variables were described as frequency and percentage.
Ordinal polytomous regressions using an odds ratio (OR) model and the Link Logit function were performed to evaluate the cross-sectional association between the independent variables and KPS (value at study’s baseline). All factors with a p-value ≤0.20 in the univariate analysis were included in the multivariate analysis.
In order to verify the KPS trajectory in the last month of life according to primary tumor site, longitudinal graphic analyzes were performed followed by the Kruskal-Wallis test with Bonferroni, comparing KPS by tumor site week by week during the last month of life.
RESULTS
Data of 108 patients with terminal cancer in palliative care who died at the PCU were evaluated, most of them females (62.0%), >60 years old (68.5%). The most prevalent primary tumor sites were GIT (24.3%), followed by breast (18.7%) and HN (16.8%), with distant metastatic disease (78.7%) who had already undergone previous oncologic treatment (82.4%) (Table 1).
Table 2 shows the most significant predictors identified by ordinal logistic regression analysis to discriminate the KPS. Only primary tumor sites remained associated with KPS [lung OR: 0.12 (95% CI: 0.03-0.67) and CNS OR: 0.13 (95% CI: 0.07-0.34)] regardless of the sex.
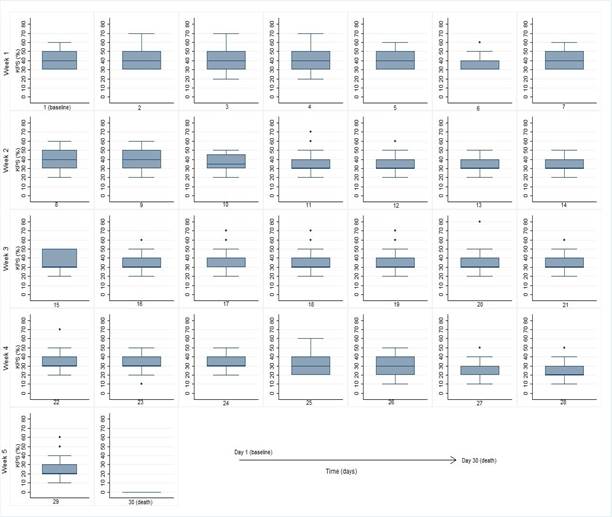
As anticipated, a decreasing median KPS was found until the date of death. At the beginning of the follow-up (day 30 before death) the most frequent KPS was 40%, remaining stable in six of the first seven days of the study. In the second, third and most of the fourth week (until the 4th day before death), KPS of 30% was more frequent. This was followed by KPS of 20%, until the day of death (KPS 0) (Figure 1).
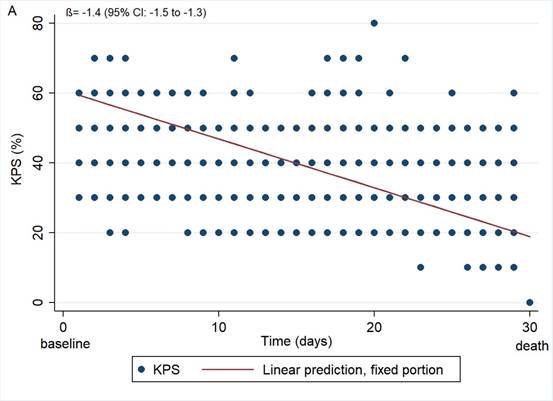
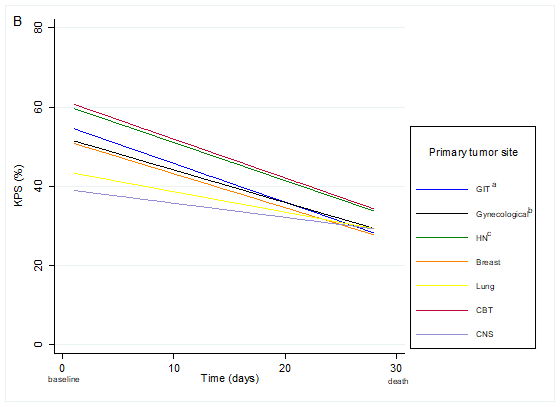
According to the graphical analysis, the reduction in KPS was more pronounced among patients with tumor primary site located in the GIT, HN, and CBT. These patients started their last month of life with higher values than the other patients. In contrast, those with primary malignant neoplasm located in CNS and lung, started the follow-up period with lower KPS values and had less flagrant reduction than the others (Graph 1 and Table 3).
|
Table 1. Demographic, clinical, and nutritional characteristics of patients with terminal cancer in palliative care at study’s baseline (n=108) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Captions: n = number of patients; % = frequency; SD = standard deviation; GIT = gastrointestinal tract; HN = head and neck; CBT = connective bone tissue; CNS = central nervous system. (a) Upper and lower GIT; (b) Oral and nasal cavity, pharynx, larynx, salivary glands, paranasal sinuses, eyes, thyroid; (c) Cervix, endometrium, ovary, vulva, and vagina; (d) Central nervous system, kidney and urinary tract, male genital organs, peritoneum, mediastinum, hematological and unrecognized site; (e) Variable with missing values; (f) Nutritional risk according to total numerical score of PG-SGA short form >9 points.
|
|
Table 2. Factors associated with Karnofsky Performance Status of patients with terminal cancer in palliative care at study’s baseline (n=108) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Captions: OR = odds ratio; CI = confidence interval; GIT = gastrointestinal tract; HN = head and neck; CBT = connective bone tissue; CNS = central nervous system. (*) p-value refers to ordinal polytomous regression; (a) Upper and lower GIT; (b) Oral and nasal cavity, pharynx, larynx, salivary glands, paranasal sinuses, eyes, thyroid; (c) Cervix, endometrium, ovary, vulva, and vagina; (d) Central nervous system, kidney and urinary tract, male genital organs, peritoneum, mediastinum, hematological and unrecognized site.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
Figure 1. Karnofsky Performance Status in the last 30 days of life of patients with terminal cancer (n=108; observations of KPS=1,120) |
|
|
|
Graph 1. Karnofsky Performance Status in patients with terminal cancer in the last 30 days in the total sample (A) and according to primary tumor site (B) (n=108; observations of KPS=1,120) Captions: GIT = gastrointestinal tract; HN = head and neck; CBT = connective bone tissue; CNS = central nervous system. (a) Upper and inferior GIT; (b) Oral and nasal cavity, pharynx, larynx, salivary glands, paranasal sinuses, eyes, thyroid; (c) Cervix, endometrium, ovary, vulva and vagina. |
|
Table 3. Median of Karnofsky Performance Status in patients with terminal cancer in the last 30 days of life by primary tumor site (n=108; observations of KPS=1,120) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Captions: KPS = Karnofsky Performance Status; GIT = gastrointestinal tract; HN = head and neck; CBT = connective bone tissue; CNS = central nervous system. (a) Upper and lower GIT; (b) Oral and nasal cavity, pharynx, larynx, salivary glands, paranasal sinuses, eyes, thyroid; (c) Cervix, endometrium, ovary, vulva and vagina; (d) Kidney and urinary tract, male genital organs, peritoneum, mediastinum, hematological and unrecognized site. Statistically significant difference of patients with tumors: (d) GIT, (e) Breast, (f) HN, (g) Gynecological, (h) Lung, (i) CBT, (j) Others. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DISCUSSION
According to the study’s findings, the median KPS decreased until death and remained associated with primary tumor sites. Thus, a patient with a CNS or lung tumors may have a lower KPS for a significant period, but not the same prognosis of another patient, for example, with the primary tumor sites in GIT, CBT and HN.
The KPS represents a global assessment of the patient's functionality, easy to use and by the entire multiprofessional team, favoring its clinical utility. Among the validated and subjective functionality assessment tools [KPS, Palliative Performance Scale (PPS) and Eastern Cooperative Oncology Group (ECOG)], KPS has the best inter-rater agreement8.
Prognostic-based decision-making depends on the ability to estimate survival as accurately as possible, which remains a challenge for healthcare professionals. Understanding the factors associated with KPS and its evolution in each type of cancer will directly help the care process, favoring individualized and patient-centered care, due to its predictive power9. It is necessary that the patients’ needs with terminal cancer are met, promoting the quality of death, reducing the inappropriate use of futile strategies, and contributing for the efficient use of resources10-12.
There are scarce longitudinal studies in the literature investigating terminal cancer patients in palliative care, not only related to functionality, usually due to the expressive rates of follow-up loss, either by functional worsening, deterioration of cognition, intensification of symptoms and complex methodological/ethical issues.
Jang et al.13 developed a study investigating patients with advanced cancer using different tools to assess functional capacity and its predictive power. In comparison with the current study, this research showed similar KPS values near the last month of life. The median survival times were: KPS 80 to 100 = 215 days; KPS 60 to 70 = 119 days; KPS 40 to 50 = 49 days; and KPS 10 to 30 = 29 days.
According to Rosa et al.14 based on patient data from the same institution, KPS was a strong prognostic factor for reduced survival in hospitalized patients, with KPS 30-40% being associated with worse survival. In this context, this work adds evidence that the KPS value for each primary tumor site may represent a different prognosis in the last month of life. Most likely, these data provide elements to improve prognostic assessment and care planning.
Although most patients had distant metastasis15,16, nutritional risk17, no association was found between these and functionality, even though they are widely recognized as prognostic factors in cancer. Thus, it is hypothesized that these factors are not associated in the context of the last days of life. As for the previous treatment, the absence of association with functionality can be attributed because it is a heterogeneous sample.
Studies investigating changes in patient functionality in the last month of life are extremely rare, especially when considering the primary tumor site. Patients with primary tumor in GIT, CBT and HN presented decline of KPS more pronounced like the results shown by Valle et al.18. In this context, Lee et al.19 observed that the location of the neoplasm in the GIT was a factor associated with survival time.
The current findings also showed that patients with primary malignant neoplasms located in CNS and lung had less flagrant reduction of KPS, maintaining not only lower but also more stable values throughout the period. In a retrospective study of lung cancer patients, the main causes of hospitalization in PCU were performance status and dyspnea deterioration20. In this way, it is possible to attribute these symptoms to poor KPS in the last month of life. On the other hand, it is known that patients with advanced stage CNS tumors often have drowsiness and cognitive impairment, which affects the KPS21. Thus, it is essential to consider in these types of cancer other more specific clinical signs able to indicate death´s proximity. In patients with glioblastoma, it is a challenge to evaluate signs of symptoms that precede death, due to the drop in the level of consciousness and worsening of the level of cognition22.
Furthermore, the choice of 2019 as the data extraction period was to avoid COVID-19 pandemic related biases, which started in 2020 in Brazil. One of the main limitations of the study is the small sample size, but values with statistical significance were obtained. In addition, the findings may have limited the generalizability of the data, as it was performed in a single institution. More studies should be developed to improve the scientific evidence in this context, with larger samples, in different settings and evaluating other associated factors.
CONCLUSION
KPS decreased until the time of death with different intensity according to the primary tumor site in patients with terminal cancer. If the primary tumor site is not considered, it is possible that the prognostic assessment will be less accurate.
CONTRIBUTIONS
Livia Costa de Oliveira, Karla Santos da Costa Rosa, Andrezza Helena Regadas Muniz contributed for the study design and conception; Livia Costa de Oliveira and Andrezza Helena Regadas Muniz contributed for the acquisition and analysis of the data; Livia Costa de Oliveira, Karla Santos da Costa Rosa and Simone Garruth dos Santos Machado Sampaio, contributed for the interpretation of the data; Livia Costa de Oliveira, Karla Santos da Costa Rosa, Andrezza Helena Regadas Muniz, Juliana Miranda Dutra de Resende and Simone Garruth dos Santos Machado Sampaio drafted the manuscript. All the authors have revised the manuscript and approved the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
None.
REFERENCES
1. Neeman E, Gresham G, Ovasapians M, et al. Comparing physician and nurse Eastern Cooperative Oncology Group Performance Status (ECOG-PS) ratings as predictors of clinical outcomes in patients with cancer. Oncologist. 2019;24(12):e1460-e6. doi: https://doi.org/10.1634/theoncologist.2018-0882
2. Sanvezzo VMS, Montandon DS, Esteves LSF. Instrumentos de avaliação de funcionalidade de idosos em cuidados paliativos: uma revisão integrativa. Rev Bras Geriatr Gerontol. 2018;21(5):627-38. doi: https://doi.org/10.1590/1981-22562018021.180033
3. Pereira EEB, Santos NB, Sarges ESNF. Avaliação da capacidade funcional do paciente oncogeriátrico hospitalizado. Rev Pan-Amaz Saude. 2014;5(4):37-44. doi: https://doi.org/10.5123/S2176-62232014000400005
4. Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM. Evaluation of chemotherapeutic agents. New York: Columbia Univ Press; 1949. p. 191-205.
5. Rosa KSC, Oliveira AS, Cypriano RP. Prognostic factors in inpatients with advanced cancer at a palliative care unit. Braz J Oncol. 2022;18:e-20220344. doi: https://doi.org/10.5935/2526-8732.20220344
6. PG-SGA/Pt-Plataforma Global [Internet]. Vernon Hills: Pt-Global; c2014 – [cited 2022 Feb 2]. Available from: https://pt-global.org/
7. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12(1 Suppl):S15-9. doi: https://doi.org/10.1016/0899-9007(96)90011-8
8. Chow R, Bruera E, Temel JS, et al. Inter-rater reliability in performance status assessment among healthcare professionals: an updated systematic review and meta-analysis. Support Care Cancer. 2020;28(5):2071-8. doi: https://doi.org/10.1007/s00520-019-05261-7
9. Perez-Cruz PE, Shamieh O, Paiva CE, et al. Factors associated with attrition in a multicenter longitudinal observational study of patients with advanced cancer. J Pain Symptom Manage. 2018;55(3):938-45. doi: https://doi.org/10.1016/j.jpainsymman.2017.11.009
https://doi.org/10.1590/1983-80422022301510PT
11. Paiva et al. Prognostication in advanced cancer: update and directions for future research. Support Care Cancer. 2019;27(6):1973-84. doi: https://doi.org/10.1007/s00520-019-04727-y
12. Mori M, Morita T, Bruera E, et al. Prognostication of the last days of life. Cancer Res Treat. 2022;54(3):631-43. doi: https://doi.org/10.4143/crt.2021.1573
13. Jang RW, Caraiscos VB, Swami N, et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10(5):e335-41. doi: https://doi.org/10.1200/JOP.2014.001457
14. Rosa KSC, Cyipriano RP, Albuquerque NM, et al. Predictive factors of death on hospitalization in patients with advanced cancer in palliative care. Am J Hosp Palliat Care. 2021;38(10):1189-94. doi: https://doi.org/10.1177/1049909120976398
15. Yang J, Peng A, Wang B, et al. The prognostic impact of lymph node metastasis in patients with non-small cell lung cancer and distant organ metastasis. Clin Exp Metastasis. 2019;36(5):457-66. doi: https://doi.org/10.1007/s10585-019-09985-y
16. Min Y, Liu X, Hu D, et al. Risk factors, prognostic factors, and nomogram for distant metastasis in breast cancer patients without lymph node metastasis. Front Endocrinol (Lausanne). 2021;24;12:771226. doi: https://doi.org/10.3389/fendo.2021.771226
17. Santos I, Mendes L, Mansinho H, et al. Nutritional status and functional status of the pancreatic cancer patients and the impact of adjacent symptoms. Clin Nutr. 2021;40(11):5486-93. doi: https://doi.org/10.1016/j.clnu.2021.09.019
18. Valle TD, Turrini RNT, Poveda VB. Fatores intervenientes para o início do tratamento de pacientes com câncer de estômago e colorretal. Rev Latino-Am Enfermagem. 2017;25:e2879. doi: https://doi.org/10.1590/1518-8345.1493.2879
19. Lee GJ, Gwak JH, Kim MS, et al. Changes in the palliative performance scale may be as important as the initial palliative performance scale for predicting survival in terminal cancer patients. Palliat Support Care. 2021;19(5):547-51. doi: https://doi.org/10.1017/S1478951520001248
20. Masel EK, Schur S, Nemecek R, et al. Palliative care units in lung cancer in the real-world setting: a single institution's experience and its implications. Ann Palliat Med. 2017;6(1):6-13. doi: https://doi.org/10.21037/apm.2016.08.06
21. Barz M, Gerhardt J, Bette S, et al. Prognostic value of tumour volume in patients with a poor Karnofsky performance status scale - a bicentric retrospective study. BMC Neurol. 2021;21(1):446. doi: https://doi.org/10.1186/s12883-021-02424-0
22. Thier K, Calabek B, Tinchon A, et al. The Last 10 Days of patients with glioblastoma: assessment of clinical signs and symptoms as well as treatment. Am J Hosp Palliat Care. 2016;33(10):985-8. doi: https://doi.org/10.1177/1049909115609295
Recebido em 20/6/2022
Aprovado em 31/10/2022
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde