ORIGINAL ARTICLE
Pain Assessment in Pediatric Patients with Primary Bone Cancer in a Single Site Cohort
Avaliação da Dor em Pacientes Pediátricos com Câncer Ósseo Primário em uma Coorte de um Centro Único
Evaluación del Dolor en Pacientes Pediátricos con Cáncer Óseo Primario en una Cohorte de un Solo Centro
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n1.3299
1Instituto Nacional de Câncer (INCA), Hospital Federal dos Servidores do Estado. Rio de Janeiro (RJ), Brazil. E-mail: andradefferreira@gmail.com. Orcid iD: https://orcid.org/0000-0001-5162-9480
2Universidade Federal de São Carlos (UFSCar). São Paulo (SP), Brazil. E-mail: cristina.ortiz@ufscar.br. Orcid iD: https://orcid.org/0000-0002-6925-4346
3INCA. Rio de Janeiro (RJ), Brazil. E-mail: sferman@uol.com.br. Orcid iD: https://orcid.org/0000-0002-7076-6779
4Universidade Federal Fluminense (UFF). Niterói (RJ), Brazil. E-mail: aricardo@id.uff.br. Orcid iD: https://orcid.org/0000-0002-3896-9226
Corresponding author: Flavio Ferreira de Andrade. Departamento de Pediatria Oncológica do INCA. Praça Cruz Vermelha, 23, 5º andar – Centro. Rio de Janeiro (RJ), Brazil. CEP 20230-130. E-mail: andradefferreira@gmail.com
ABSTRACT
Introduction: Pain is the main symptom described in cancer patients. Objective: To assess pain classification and management in pediatric patients with primary bone cancer over time: admission, during treatment and follow-up, and to investigate factors associated with pain classification at the last assessment. Method: Retrospective cohort study of osteosarcoma and Ewing's sarcoma cases in individuals <19 years old treated at a single cancer referral site and followed up by a multidisciplinary team. The primary endpoint was pain score at the last assessment. Secondary outcome: evolution of pharmacological treatment. Results: 142 patients were included. The frequency of pain assessment increased during the study period from 53.5% at admission to 68.3% during treatment and 85.9% in follow-up. Of the patients who had pain assessed, 65.8% had pain at admission and 26.2% at the end of the study. There was an increase in the use of strong opioids and antidepressants. In the last evaluation, 56 patients (39.4%) were at the end-of-life and this was not associated with more pain (p=0.68). Meanwhile, those who had more pain used strong opioids (p=0.01) or steroids (p=0.03). Conclusion: Pain management during treatment resulted in increased use of strong opioids and antidepressants with pain reduction, revealing that pain control is possible. In the last assessment, end-of-life patients no longer had pain and patients with pain were the ones who used strong opioids and steroids at the most, showing the difficulty of pain control in some patients.
Abstrac
Key words: sarcoma, Ewing; osteosarcoma; pain management; death; bone neoplasms.
RESUMO
Introdução: A dor é o principal sintoma descrito em pacientes com câncer. Objetivo: Avaliar a classificação e o manejo da dor em pacientes pediátricos com câncer ósseo primário ao longo do tempo: admissão, durante o tratamento e acompanhamento, e investigar fatores associados à classificação da dor na última avaliação. Método: Estudo de coorte retrospectivo de casos de osteossarcoma e sarcoma de Ewing em indivíduos <19 anos, atendidos em único centro de referência de câncer e acompanhados por equipe multidisciplinar. Desfecho primário: classificação da dor na última avaliação. Desfecho secundário: evolução do tratamento farmacológico. Resultados: Foram incluídos 142 pacientes. A frequência de avaliação da dor aumentou durante o período do estudo de 53,5% na admissão para 68,3% durante o tratamento, chegando a 85,9% no acompanhamento. Dos pacientes cuja dor foi avaliada, 65,8% tiveram dor no recrutamento e 26,2% ao final do estudo. Houve aumento no uso de opioides fortes e antidepressivos. Na última avaliação, 56 pacientes (39,4%) estavam no fim da vida sem associação com mais dor (p=0,68), enquanto os que apresentaram mais dor foram aqueles que usavam opioides fortes (p=0,01) ou esteroides (p=0,03). Conclusão: O manejo da dor durante o tratamento resultou em aumento do uso de opioides fortes e antidepressivos com redução da dor, revelando que o controle da dor é possível. Na última avaliação, os pacientes em fim de vida não apresentavam mais dor, e os pacientes com dor foram os que mais utilizaram opioides fortes e esteroides, evidenciando a dificuldade no controle da dor em alguns pacientes.
Palavras-chave: sarcoma de Ewing; osteossarcoma; manejo da dor; morte; neoplasias ósseas.
RESUMEN
Introducción: El dolor es el principal síntoma descrito en pacientes oncológicos. Objetivo: Evaluar la clasificación y el manejo del dolor en pacientes pediátricos con cáncer óseo primario a lo largo del tiempo: registro, durante el tratamiento y seguimiento, e investigar los factores asociados entre la clasificación del dolor y última evaluación. Método: Estudio cohortes retrospectiva de casos de osteosarcoma y sarcoma de Ewing <19 años, tratados en único centro de referencia oncológica y seguidos por equipo multidisciplinar. Desenlace primario: calificación del dolor en la última evaluación. Desenlace secundario: evolución del tratamiento farmacológico. Resultados: Se incluyeron 142 pacientes. La frecuencia de evaluación del dolor aumentó durante el período de estudio del 53,5% al 68,3% y 85,9%. Los pacientes evaluados por dolor, el 65,8% tenía dolor al registro y 26,2% al final del estudio. Hubo aumento en el uso de opioides fuertes y antidepresivos. En la última evaluación, 56 pacientes (39,4%) estaban al final de su vida y esto no se asoció con más dolor (p=0,68), mientras que, quienes presentaron más dolor fueron quienes usaban opioides fuertes (p=0,01) o esteroides (p=0,03). Conclusión: Manejo del dolor durante el tratamiento resultó en un mayor uso de opioides fuertes y antidepresivos con reducción del dolor, revelando que es posible controlar el dolor. La última evaluación, pacientes al final de la vida ya no tenían dolor y pacientes con dolor eran los que más usaban opioides fuertes y esteroides, evidenciando la dificultad en el control del dolor en algunos pacientes.
Palabras clave: sarcoma de Ewing; osteosarcoma; manejo del dolor; muerte; neoplasias óseas.
INTRODUCTION
The National Cancer Institute (INCA)1 estimated that from 2023 to 2025 there will be 704,000 new patients with cancer in Brazil. Of these, 7,930 will be children and adolescents and 5% with bone cancer, either osteosarcomas (OS) or Ewing’s sarcomas (ES). Pain is the most frequent symptom in these patients, present in 75% to 90% of advanced cases2-5, respectively. Also, pain can be present from the moment of the diagnosis until death6-8.
With advances in pediatric bone cancer treatment, especially the use of a combination of chemotherapies and supportive care, death rates have been declining9. However, pain has been an important factor influencing their quality-of-life. Special attention has been given to patients with progressive disease or in end-of-life10,11.
There are several pain management guidelines, as the World Health Organization (WHO) and Expert Working Group of European Association for Palliative Care’s recommendations. However, even with adequate treatment some patients report pain and pain undertreatment is still a problem for up to 40% of patients with advanced cancer, one possible reason is the inappropriate use of opioids12,13.
Considering this scenario, the aim of the study was to evaluate pain management in children and adolescents with primary bone cancer at admission, during treatment and follow up and to investigate factors associated with the classification of pain symptom at the last evaluation.
METHOD
Medical charts of children and adolescents under 19 years treated for OS or ES at INCA, between January 2011 and December 2016 were retrospectively reviewed after local Institutional Review Board (IRB) approval (CAAE: 67729317.5.0000.5274, report number 2113448, 2017); because it is a retrospective study, the Informed Consent Form was waived. INCA is the national reference center for cancer patients and receives patients from every Brazilian region. Patients with unavailable medical records and those admitted only for radiotherapy and who underwent cancer treatment at another hospital were excluded. INCA’s pathology specialists routinely reviewed all the cases. Patients were registered either in the Latin American Cooperative Group for Osteosarcoma Protocol of Treatment (GLATO) or in the Latin American Cooperative Group for Ewing Tumors Family (GALOP)14,15. Considering a population of 157 patients with primary bone cancer and pain prevalence of 25%, α=0.05 and 95% confidence, a sample of 142 patients was obtained.
Three moments were observed during this cohort: at admission, three months later and at the last evaluation before the end of the study (December 31st, 2019) or death, whichever occurred first. Patients were considered at end-of-life when last evaluation occurred up to one month before death16,17. Patients alive at the end of study could be on treatment or in disease control and were declared survivors. To assess the pain, all health professionals at INCA were regularly trained to be able to apply the pain scales which are normally used by the Pediatric Oncology Department. The measurement of pain assessment was recorded and classified according to the Wong-Baker scale, where 0 is no pain; 1 to 3, mild pain, 4 to 6, moderate pain, 7 to 9, severe pain and 10, excruciating pain18.
The primary endpoint was pain classification (yes/no) at the last evaluation. Covariables were age at diagnosis, sex, race, cancer diagnosis (OS or ES), presence of metastases at diagnosis, progressive disease, pain medication used (mild analgesics, weak opioids, strong opioids, anticonvulsants, antidepressants, and steroids) and end-of-life patient (yes/no). This study followed STROBE’s (Strengthening the Reporting of Observational Studies in Epidemiology) recommendations.
Care coordination and pain management at the Pediatric Oncology Department
Aware that pain is a common and highly distressing symptom in pediatric patients with cancer and advanced malignancies, strategies were developed at the Pediatric Oncology Department to reduce pain and improve quality-of-life. A pain service was created in 1999, coordinated by one pediatric oncologist, specialized in pediatric pain management following a multidisciplinary approach with nurses and social workers. Since then, the oncology team has referred all patients with pain or advanced disease to be followed by specialists at the ward or as outpatients.
The pediatric palliative care service was created at the department in 2008. There was close collaboration between palliative care and pain specialists, especially in end-of-life patients.
Pharmacological pain management is made according to institutional pain protocols. If patients were already using a medication for pain control, it was modified whenever necessary, according to the multidisciplinary evaluation. Protocols included the prescription of mild analgesic plus an adjuvant for mild pain, mild analgesic plus weak opioid and an adjuvant for moderate pain and the combination of a strong opioid and an adjuvant for severe and excruciating pain. Nonpharmacological pain management included psychosocial interventions, physical therapy, occupational therapy and play therapy, all of them patient-centered according to clinical condition and to their desires and preferences.
Stata 13.0 (Stata Corp, LC) software was used for statistical analysis. The Kolmogorov-Smirnov test was used to test normality. Results are presented as median, interquartile range (IQR), frequencies and 95% confidence intervals (95% CI). Patients’ characteristics and cross-sectional pain assessment (at admission, three months later and at last evaluation) were evaluated. Differences between medians were evaluated by Mann Whitney test. Differences between categorical variables were evaluated by Fisher exact test and differences between three paired proportions were evaluated by Q Cochran test. To identify possible associations among variables and pain classification at the last evaluation, a Poisson regression analysis with robust estimation and log link function to determine crude prevalence ratios (CPR) was calculated; patients’ demographic characteristics, presence of metastasis at admission, progressive disease at last evaluation, end-of-life (yes/no) and the use of mild analgesics, weak or strong opioids at last evaluation. Variables associated with pain (CPR with p value<0.20) were included in a multivariate model to determine adjusted prevalence ratios (APR). A p value of <0.05 was considered statistically significant.
RESULTS
Of the 917 patients enrolled at INCA during the study period, 157 were children and adolescents with primary bone cancer, 15 of which (9.6%) had no medical records available and were excluded. To analyze the possibility of bias caused by the exclusions, these patients were compared to the remaining cohort sample and no differences according to age (p=0.42), diagnosis (p=0.30) and survival (p=0.24) were found. Data of the patients excluded were not considered in the cohort analysis.
The study enrolled 142 patients, with median age of 13 years (IQR 9-14 years), 43.6% were males, 99 (69.7%) with OS and 43 (30.3%) with ES. Seventy-two (50.7%) patients had metastatic disease at admission. Patients’ characteristics at admission are in Table 1.
|
Table 1. Demographic and disease characteristics of children and adolescents with primary bone cancer at admission (n=142) |
||||||||||||||||||
|
||||||||||||||||||
|
Captions: CI = confidence interval; OS = osteosarcoma; ES = Ewing’s sarcoma. |
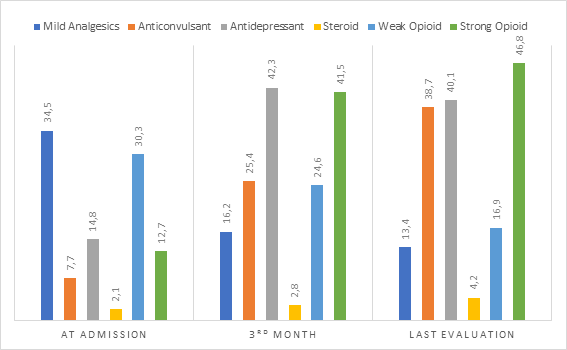
Considering pain treatment with medications at admission, three months later and at last evaluation there was an increase of the use of strong opioids from 12.7% to 46.8% (p<0.001), antidepressants from 14.8% to 40.1% (p<0.001) and a reduction of the use of mild analgesics from 34.5% to 13.4% (p<0.001) (Chart 1).
|
|
|
Chart 1. Frequency (%) of children and adolescents with primary bone cancer who used different classes of medications for pain management during follow up (n=142) |
The comparison of pain assessment at admission, three months later and at the last evaluation revealed that pain assessment rate increased during treatment. 122 patients (85.9%) had pain assessed at the last evaluation. Also, the proportion of patients without pain ranged from 34.2% to 77.3% and reached 73.8% in the three timepoints of evaluation during the study (p<0.001) and excruciating pain was not referred in the last evaluation (Table 2).
|
Table 2. Pain assessment and classification in children and adolescents with primary bone cancer at admission, three months later and at the last evaluation (n=142) |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
(#) According to the Wong-Baker scale. (*) p<0.05 by Q Cochran test. |
||||||||||||||||||||||||||||
Fifty-six (39.4%) patients evolved to death. The frequency of pain was 25% for end-of-life patients and 22.0% among survivors at the last evaluation. End-of-life patients compared with the survivors in this study used more frequently strong opioids (53.5% vs 41.3%) and anticonvulsants (42.8% vs 36.0%) at the last evaluation.
A Poisson regression analysis to estimate CPR of pain classification (yes/no) at the last evaluation revealed no difference between end-of-life patients and the survivors (p=0.68). All variables with p value<0.20 were tested in a multivariate model to predict APR (Table 3).
|
Table 3. Univariate and multivariate Poisson regression for the predictors of pain complaint (yes/no) and variables at the last evaluation (n=142) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Captions: CPR = crude prevalence ratio; APR = adjusted prevalence ratio; CI = confidence interval. (*) p-value associated with Poisson regression. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DISCUSSION
Pain control in cancer patients is a challenge and quality-of-life is affected by the management of this symptom10. Anghelescu et al.19 observed that pain assessment influenced the effectiveness of the pain control in end-of-life children with cancer, leading to the necessity to adjust pain relief medications, principally the use of strong opioids19. In comparison with this study, pain assessment had increased and impacted the pain relief, even if the goal should be to have pain assessment for all the patients since diagnosis. These results suggest the effectiveness of pain control management during follow-up.
Madden et al.11 investigated all children with cancer, 86.0% of them reported pain. A systematic review of self-reported pain in childhood cancer survivors found that the highest prevalence of pain (23.0%) occurred in those with bone cancer, revealing the importance of this issue20. This study did not compare self-reported pain and classified pain according to the Wong-Baker scale18.
At admission, the most frequently drugs used for pain control were mild analgesics (34.5%) and three patients had excruciating pain, which might be suggestive of pain undertreated, in contrast with WHO’s recommendation, which reinforced that opioids are fundamental in pain management21. Friedrichsdorf22 commented that many distressing symptoms in children with advanced cancer, including pain, were not effectively treated. Also, the author emphasized that pain treatment needed to be individualized, frequently evaluated, and modified as required.
Pharmacological treatment must be the cornerstone of pain management in these children, but it has been suggested that many physicians and patients’ families resist in using opioids23. Edmonds et al.24 reaffirmed that methadone is an important drug in the treatment of pediatric cancer-related pain, it has the advantage of being effective for both nociceptive and neuropathic pain and its use can be safe.
During follow up, the use of strong opioids and antidepressants increased and mild analgesics declined, in concurrence with the recommendations21-23,25, as treatment algorithms applied to children with cancer include antidepressants, optimizing the pain management, targeted to minimize neuropathic pain, frequently present in these circumstances21-23,25.
At the last evaluation, patients with pain used 2.5 times more strong opioids and 3.9 times more steroids. This association does not imply in a cause-effect relationship. Instead, it signals that those who had pain were already using more strong opioids or steroids, revealing concerns about pain control21,25.
Those patients continued to be followed by pain clinic specialists after the last study evaluation with pharmacological treatment adjustments concurring with results reached by Anghelescu et al.19, who revealed that over time, the demand for opioids and adjuvant medications increased. In contrast with this study, it compared different doses of medications.
In Chile, Fernández Urtubia et al.23 analyzed children with advanced cancer in a case-series study and they noticed that 77% of the patients received some adjuvant medication and more than a half steroids. Primary bone cancer patients in different disease stages increased the use of steroids since admission to the last evaluation (0.7% to 4.2%). It is possible that dose adjustment and opioid rotation could have controlled pain in those patients. Also, nonpharmacologic strategies for pain control were not controlled in the present study but it is acknowledged that they constitute the multimodal integrative approach recommended by the Centers for Disease, Control and Prevention (CDC) for children and adolescents with cancer26.
No differences in pain between end-of-life patients and the survivors at the last evaluation was found, suggesting that it is possible to give these patients a better quality-of-life by pain relief. Although all end-of-life patients were in palliative care, the results are interesting and intriguing, because they seem lower than the reported by other authors10,19,27,28.
Unlike the current investigation, they analyzed end-of-life patients without a control group. Wolfe at al. reported that 76% of the dying children with cancer were treating pain unsuccessfully in less than 30% as informed by their parents27. Lykke et al.28 also reported physical fatigue in 93%.
These studies, with different methodologies, reported dissimilar results, showing the difficulty in comparing studies and highlighting the importance of pain management28. Again, all patients were end-of-life without control group. Pritchard et al.29 investigated the symptoms of most concern reported by parents of dying children and they revealed that mood changes, breathing and pain were the most frequent. Only 14.6% of these parents reported that pain was a concerning symptom in the dying children. Protocols for treatment of chronic neuropathic and mixed pain in children that included opioids and adjuvant medications, such as gabapentin, have been studied and these drugs are in current clinical use19,25,30.
A single center is a limitation of the study because it reflects a specific local setting in managing pain in children and adolescents with primary bone cancer. It is possible that some information failed to be reported in the charts and medications doses were not registered in the study protocol. The strength of the study relies on the sample size formed by patients with primary bone cancer, and the robust multivariable analysis. Prospective, longitudinal-based studies are necessary to explore pain assessment, pain classification and temporal changes in treatment of end-of life patients, compared to survivors.
CONCLUSION
This study revealed that optimizing pain management during cancer treatment with strong opioids and antidepressants and that pain control is possible in children and adolescents with primary bone cancer. End-of-life patients did not have more pain, when compared to the survivors. Patients with pain at the last evaluation were using more frequently strong opioids and steroids, showing the difficulty to control pain in some patients, that may be resistant regardless of all pain management approaches.
ACKNOWLEDGMENTS
To all the patients and to the multiprofessional team who cared for them.
CONTRIBUTIONS
Flavio Ferreira de Andrade participated in the study design, data collection, analysis and interpretation, wording, manuscript, critical review. Cristina Ortiz Sobrinho Valete participated in the study conception, data analysis and interpretation, wording, critical review. Sima Ferman participated in the study conception, wording, data interpretation, critical review. André Ricardo Araújo Silva participated in the wording, critical review, data interpretation. All the authors approved the final version published.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
None.
REFERENCES
1. Instituto Nacional de Câncer. Estimativa 2023: incidência de câncer no Brasil [Internet]. Rio de Janeiro: INCA; 2022 [acesso 2023 fev 14]. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2023.pdf
2. Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353(9165):1695-700. doi: https://doi.org/10.1016/S0140-6736(99)01310-0
3. van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, et al. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070-1090.e9. doi: https://doi.org/10.1016/j.jpainsymman.2015.12.340
4. Wiffen PJ, Wee B, Derry S, et al. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;7(2):CD012592. doi: https://doi.org/10.1002/14651858.CD012592.pub2
5. Mikan F, Wada M, Yamada M, et al. the association between pain and quality of life for patients with cancer in an outpatient clinic, an inpatient oncology ward, and inpatient palliative care units. Am J Hosp Palliat Care. 2016;33(8):782-90. doi: https://doi.org/10.1177/1049909116630266
6. Marec-Bérard P, Delafosse C, Foussat C. Douleurs et tumeurs osseuses malignes de l'enfant et de l'adolescentCancer-related bone pain in children. Arch Pediatr. 2005;12(2):191-8. doi: https://doi.org/10.1016/j.arcped.2004.11.026
7. Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. IASP task force on cancer pain. International Association for the study of pain. Pain. 1999;82(3):263-74. doi: https://doi.org/10.1016/S0304-3959(99)00073-1
8. Widhe B, Widhe T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J Bone Joint Surg Am. 2000;82(5):667-74. doi: https://doi.org/10.2106/00004623-200005000-00007
9. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. In: Jaffe N, Bruland OS, Bielack S, editors. Pediatric and adolescent osteosarcoma. Boston (MA): Springer; 2009. p. 3-13. doi: http://link.springer.com/10.1007/978-1-4419-0284-9_1
10. Zernikow B, Szybalski K, Hübner-Möhler B, et al. Specialized pediatric palliative care services for children dying from cancer: a repeated cohort study on the developments of symptom management and quality of care over a 10-year period. Palliat Med. 2019;33(3):381-91. doi: https://doi.org/10.1177/0269216318818022
11. Madden K, Magno Charone M, Mills S, et al. Systematic symptom reporting by pediatric palliative care patients with cancer: a preliminary report. J Palliat Med. 2019;22(8):894-901. doi: https://doi.org/10.1089/jpm.2018.0545
12. Deandrea S, Montanari M, Moja L, et al. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-91. doi: https://doi.org/10.1093/annonc/mdn419
13. Maltoni M. Opioids, pain, and fear. Ann Oncol. 2008;19(1):5-7. doi: https://doi.org/10.1093/annonc/mdm555
14. Senerchia AA, Macedo CR, Ferman S, et al. Results of a randomized, prospective clinical trial evaluating metronomic chemotherapy in nonmetastatic patients with high-grade, operable osteosarcomas of the extremities: a report from the Latin American Group of Osteosarcoma Treatment. Cancer. 2017;123(6):1003-10. doi: https://doi.org/10.1002/cncr.30411
15. Becker RG, Gregianin LJ, Galia CR, et al. What is the impact of local control in Ewing sarcoma: analysis of the first Brazilian collaborative study group - EWING1. BMC Cancer. 2017;17(1):420. doi: https://doi.org/10.1186/s12885-017-3391-5
16. Snaman JM, Baker JN, Ehrentraut JH, et al. Pediatric oncology: managing pain at the end of life. Pediatr Drugs. 2016;18(3):161-80. doi: https://doi.org/10.1007/s40272-016-0168-2
17. Chi NC, Demiris G. Family caregivers’ pain management in end-of-life care: a systematic review. Am J Hosp Palliat Care. 2017;34(5):470-85. doi: https://doi.org/10.1177/1049909116637359
18. Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14(1):9-17.
19. Anghelescu DL, Snaman JM, Trujillo L, et al. Patient-controlled analgesia at the end of life at a pediatric oncology institution. Pediatr Blood Cancer. 2015;62(7):1237-44. doi: https://doi.org/10.1002/pbc.25493
20. Reinfjell T, Zeltzer L. A systematic review of self-reported pain in childhood cancer survivors. Acta Paediatr. 2020;109(1):56-70. doi: https://doi.org/10.1111/apa.14977
21. WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. Geneva: World Health Organization; 2012.
22. Friedrichsdorf SJ. Pain management in children with advanced cancer and during end-of-life care. Pediatr Hematol Oncol. 2010;27(4):257-61. doi: https://doi.org/10.3109/08880011003663416
23. Fernández Urtubia B, Trevigno Bravo A, Rodríguez Zamora N, et al. Uso de opiáceos en niños con cáncer avanzado en cuidados paliativos. Rev Chil Pediatr. 2016;87(2):96-101. doi: https://doi.org/10.1016/j.rchipe.2015.10.006
24. Edmonds KP, Saunders IM, Willeford A, et al. Emerging challenges to the safe and effective use of methadone for cancer-related pain in paediatric and adult patient populations. Drugs. 2020;80(2):115-30. doi: https://doi.org/10.1007/s40265-019-01234-6
25. Anghelescu DL, Tesney JM. Neuropathic pain in pediatric oncology: a clinical decision algorithm. Pediatr Drugs. 2019;21(2):59-70. doi: https://doi.org/10.1007/s40272-018-00324-4
26. Brown ML, Rojas E, Gouda S. A mind-body approach to pediatric pain management. Children (Basel). 2017;4(6):50. doi: https://doi.org/10.3390/children4060050
27. Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(5):326-33. doi: https://doi.org/10.1056/NEJM200002033420506
28. Lykke C, Ekholm O, Olsen M, et al. Paediatric end-of-life care - symptoms and problems: parent assessment. BMJ Support Palliat Care. 2021;bmjspcare-2021-002891. doi: https://doi.org/10.1136/bmjspcare-2021-002891
29. Pritchard M, Burghen EA, Gattuso JS, et al. Factors that distinguish symptoms of most concern to parents from other symptoms of dying children. J Pain Symptom Manage. 2010;39(4):627-36. doi: https://doi.org/10.1016/j.jpainsymman.2009.08.012
30. Leeuw TG, Mangiarini L, Lundin R, et al. Gabapentin as add-on to morphine for severe neuropathic or mixed pain in children from age 3 months to 18 years - evaluation of the safety, pharmacokinetics, and efficacy of a new gabapentin liquid formulation: study protocol for a randomized controlled trial. Trials. 2019;20(1):49. doi: https://doi.org/10.1186/s13063-018-3169-3
Recebido em 31/10/2022
Aprovado em 28/12/2022
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde