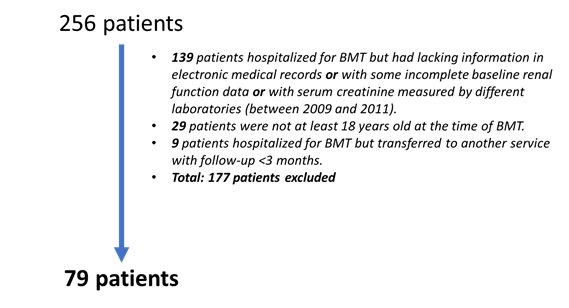Acute Kidney Injury after Bone Marrow Transplantation in Patients with Lymphomas and Leukemias
Lesão Renal Aguda após Transplante de Medula Óssea em Pacientes com Linfomas e Leucemias
Insuficiencia Renal Aguda tras Trasplante de Médula Ósea en Pacientes con Linfomas y Leucemias
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n1.3423
1,3,9Universidade Nove de Julho. São Paulo (SP), Brazil. E-mails: annacarolinamota1812@hotmail.com; gscanicoba@gmail.com; benedito.pereira@accamargo.org.br. Orcid iD: https://orcid.org/0000-0002-9886-5752; Orcid iD: https://orcid.org/0000-0002-8497-1650; Orcid iD: https://orcid.org/0000-0002-7020-4573
2,4,6-9A. C. Camargo Cancer Center, Departamento de Nefrologia. São Paulo (SP), Brazil. E-mails: joubert.alves@accamargo.org.br; germana.brito@accamargo.org.br; aline.baptista@accamargo.org.br; luis.andrade@accamargo.org.br; marina.imanishe@accamargo.org.br; benedito.pereira@accamargo.org.br. Orcid iD: https://orcid.org/0000-0001-8655-0588; Orcid iD: https://orcid.org/0000-0001-8280-1087; Orcid iD: https://orcid.org/0000-0002-6833-0482; Orcid iD: https://orcid.org/0000-0002-6301-8561; Orcid iD: https://orcid.org/0000-0002-5368-5889; Orcid iD: https://orcid.org/0000-0002-7020-4573
5A. C. Camargo Cancer Center, Departamento de Onco-hematologia. São Paulo (SP), Brazil.
E-mail: garles74@yahoo.com.br. Orcid iD: https://orcid.org/0000-0001-9326-3798
Corresponding author: Benedito Jorge Pereira. A. C. Camargo Cancer Center. Rua Professor Antônio Prudente, 211 – Liberdade. São Paulo (SP), Brazil. CEP 01509-010. E-mail: benedito.pereira@accamargo.org.br
ABSTRACT
Introduction: Hematologic malignancies, including lymphomas and leukemias, may be treated with autologous or allogeneic bone marrow transplantation. However, these approaches can increase the risk of infection, sepsis, graft-versus-host disease, and nephrotoxicity, possibly resulting in acute kidney injury (AKI). Objective: To evaluate AKI in patients with lymphomas or leukemia submitted to bone marrow transplantation (BMT). Method: Retrospective, observational cohort study of cases from a database of 256 patients (53.9% males) hospitalized for BMT between 2012 and 2014 at a cancer hospital in São Paulo, Brazil. Of these, 79 were selected randomly for analysis. Demographic data, length of hospitalization, and associated morbidities were recorded. AKI was identified according to Kidney Diseases Improving Global Outcomes (KDIGO) criteria. Results: The most frequent diagnoses for the 79 cases were non-Hodgkin’s lymphoma (30.4%), acute myeloid leukemia (26.6%), and Hodgkin’s lymphoma (24.1%). The probability of 100 days-survival after BMT was 81%, and three years after BMT was 61%. In-hospital mortality was significantly higher among patients who presented AKI during hospitalization (p<0.001). However, there was no difference in overall life expectancy (p=0.770). Conclusion: A significant prevalence of AKI was found in patients with leukemia or lymphoma while they were hospitalized for BMT, resulting in significantly increased rates of in-hospital mortality. The presence of AKI during hospitalization was not associated with a subsequent reduction in life expectancy.
Key words: leukemia; lymphoma; bone marrow transplantation; acute kidney injury.
RESUMO
Introdução: As neoplasias hematológicas, incluindo linfomas e leucemias, podem ser tratadas com transplante autólogo ou halogênico de medula óssea. No entanto, essas abordagens podem aumentar o risco de infecção, sepse, doença do enxerto contra o hospedeiro e nefrotoxicidade, possivelmente resultando em lesão renal aguda (LRA). Objetivo: Avaliar LRA em pacientes com linfomas ou leucemia submetidos a transplante de medula óssea (TMO). Método: Estudo de coorte observacional retrospectivo de casos de um banco de dados de 256 pacientes (53,9% do sexo masculino) internados por TMO entre 2012 e 2014 em um hospital oncológico de São Paulo, Brasil. Destes, 79 prontuários foram selecionados aleatoriamente para análise. Dados demográficos, tempo de internação e morbidades associadas foram registrados. A LRA foi identificada de acordo com os critérios de Kidney Diseases Improving Global Outcomes (KDIGO). Resultados: Os diagnósticos mais frequentes da amostra de 79 casos foram linfoma não Hodgkin (30,4%), leucemia mieloide aguda (26,6%) e linfoma de Hodgkin (24,1%). A probabilidade de sobrevivência em 100 dias após o TMO foi de 81% e, em três anos após o TMO, foi de 61%. A mortalidade intra-hospitalar foi significativamente maior entre os pacientes que apresentaram LRA durante a internação (p<0,001). No entanto, não houve diferença na expectativa de vida geral (p=0,770). Conclusão: Neste estudo, observou-se prevalência significativa de LRA em pacientes com leucemia ou linfoma durante a internação por TMO, resultando em aumento significativo das taxas de mortalidade intra-hospitalar. A presença de LRA durante a hospitalização não se associou a uma subsequente redução da expectativa de vida.
Palavras-chave: leucemia; linfoma; transplante de medula óssea; injúria renal aguda.
RESUMEN
Introducción: Las neoplasias malignas hematológicas, incluidos los linfomas y las leucemias, pueden tratarse con trasplante autólogo o alogénico de médula ósea. Sin embargo, estos enfoques pueden aumentar el riesgo de infección, sepsis, enfermedad de injerto contra huésped y nefrotoxicidad, lo que posiblemente provoque lesión renal aguda (IRA). Objetivo: Evaluar el FRA en pacientes con linfomas o leucemias sometidos a trasplante de médula ósea (TMO). Método: Se realizó un estudio de cohorte observacional retrospectivo de casos de una base de datos de 256 pacientes (53,9% hombres) hospitalizados por TMO entre 2012 y 2014 en un hospital de cáncer en São Paulo, Brasil. De estos, 79 registros fueron seleccionados aleatoriamente para su análisis. Se registraron los datos demográficos, la duración de la hospitalización y las morbilidades asociadas. La IRA se identificó según los criterios de Kidney Diseases Improving Global Outcomes (KDIGO). Resultados: Los diagnósticos más frecuentes en la muestra de 79 casos fueron linfoma no Hodgkin (30,4%), leucemia mieloide aguda (26,6%) y linfoma de Hodgkin (24,1%). La probabilidad de supervivencia 100 días después del BMT fue del 81% y tres años después del BMT fue del 61%. La mortalidad hospitalaria fue significativamente mayor entre los pacientes que presentaron FRA durante la hospitalización (p<0,001). Sin embargo, no hubo diferencia en la esperanza de vida global (p=0,770). Conclusión: En este estudio, se observó una prevalencia significativa de LRA en pacientes con leucemia o linfoma mientras estaban hospitalizados por TMO, lo que resultó en un aumento significativo de las tasas de mortalidad hospitalaria. La presencia de FRA durante la hospitalización no se asoció con una reducción posterior de la esperanza de vida.
Palabras clave: leucemia; linfoma; trasplante de médula ósea; lesión renal aguda.
INTRODUCTION
Leukemia is a clonal disorder of hematopoiesis characterized by recurrent chromosomal aberrations and genetic mutations that cause abnormal cell accumulation in the blood, impairing the production of red blood cells, white blood cells, and platelets1-3. Overall, leukemia is of great clinical relevance. According to the Brazilian National Cancer Institute (INCA)4, the estimated occurrence of new cases of leukemia in Brazil for each year of the triennium 2023-2025 is 11,540 cases, corresponding to an estimated risk of 5.33 per 100,000 inhabitants, being 6,250 in males and 45.9% 5,290 in females4. The morbimortality prognosis of leukemia is related to the subtype, patient age, and adverse prognostic factors, including infiltration of the central nervous system, infection at diagnosis, and hyperleukocytosis5-7.
Lymphomas are neoplastic transformations of lymphoid cells8, most of them with uncertain etiology, but it has been suggested that its development may be related to heredity, environment, occupation, and diet9,10. They are classified as either Hodgkin's (HL) or non-Hodgkin's (NHL). HL is relatively curable: chemotherapy and bone marrow transplantation (BMT) result in remission in 80% of patients. NHL presents a more challenging outlook, with a 60% 5-year overall survival prognosis and a cure rate (defined as continuous remission for 24 months) of only 50% for aggressive cases in a USA11. Likewise, Brazil’s Mortality Information System12 recorded 536 deaths by HL compared to 4,154 deaths by NHL in 2013, and INCA reported 3,080 new cases of HL with 14.7% (n=455) mortality and 12,040 new cases of NHL with a 36.1% (n=4,357) mortality4,13,14.
BMT is a potential curative treatment option for malignant and non-malignant hematological disorders15,16. Allogeneic transplantation is usually preferred in cases of NHL and most leukemias, and autologous transplantation is more often used in cases of HL. Both methods are known to increase the risk of renal lesions17,18. Renal failure, which can develop within two weeks of allogeneic transplantation, has been related to several risk factors, such as advanced age, any complication, and the gender of the patient19,20.
Transplanted patients may also experience acute kidney injury (AKI), a common complication which affects both morbidity and mortality. It is characterized by an abrupt decline in glomerular filtration rate, which manifests clinically as a sustained increase of serum creatinine and reduction of urinary volume21. Approximately 40% of the patients receiving allogeneic transplants experience AKI22.
Up to 60% of the patients with hematologic cancer experience AKI at some point in the course of the disease, most associated with sepsis, nephrotoxins, immunosuppressive agents, and graft-versus-host disease, which may affect glomerular, tubulointerstitial, and vascular structures23.
Cancer treatment regimens are becoming more complex and often involve combined therapies. Some of the recent agents introduced had few or no renal adverse effects during clinical trials, but renal injury cases emerged upon widespread use including administration to patients with a less robust baseline renal function and other comorbidities24.
Before undergoing BMT, patients are prescribed immunosuppressive therapies to support donor cell grafting and prevent graft-versus-host disease. Drug interactions may occur due to exposure to complex multidrug regimens with narrow therapeutic windows and high toxicity profiles17. Together, these treatments and vulnerabilities may accumulate to enable AKI pathogenesis. It may be helpful to administer nephroprotective drugs to at-risk BMT patients.
The primary aim of the present study was to analyze the occurrence of AKI in leukemia/lymphoma patients who underwent BMT to identify risk factors in this population. In addition, the relationship of AKI incidence with post-BMT prognosis was analyzed.
METHOD
Retrospective clinical study where the electronic medical records of patients with leukemia or lymphomas hospitalized for BMT at a Cancer Center in São Paulo, Brazil, between 2009 and 2014 were evaluated. The inclusion criteria were ≥18 years and undergoing BMT at the same hospital. The exclusion criteria were follow-up <3 months, baseline laboratory tests performed by different laboratories, incomplete baseline renal function data and Stage V chronic kidney disease (CKD) as defined by the National Kidney Foundation or having been in dialysis for >1 year.
To present the results of this study, the lymphomas were classified according to the WHO Classification, 5th edition25 among HL and all NHL: tumor-like lesions with B-cell predominance; precursor B-cell neoplasms (B-cell lymphoblastic leukemias/lymphomas); mature B-cell neoplasms (pre-neoplastic and neoplastic small lymphocytic proliferations, splenic B-cell lymphomas and leukemia); lymphoplasmacytic lymphoma; marginal zone lymphoma; follicular lymphoma; cutaneous follicle center lymphoma; mantle cell lymphoma; transformations of indolent B-cell lymphomas; large B-cell lymphomas; KSHV/HHV8-associated B-cell lymphoid proliferations and lymphomas; lymphoid proliferations and lymphomas associated with immune deficiency and dysregulation and plasma cell neoplasms and other diseases with paraproteins26.
Among the more than 12 types of leukemias found in the present investigation, four primary types were described: acute myeloid leukemia (AML), chronic myeloid leukemia (CML), acute lymphocytic leukemia (ALL) and chronic lymphocytic leukemia (CLL)27.
Pre- and post-BMT creatinine levels were extracted from electronic charts. Glomerular filtration rate and CKD stage were determined based on the CKD Epidemiology Collaboration equation. Light-chain dosages before and after BMT, chemotherapy treatments, type of BMT performed, any major complications of BMT, and demographic data (age, sex, race, and body mass index), and associated morbidities (subarachnoid hemorrhage, diabetes mellitus, liver disease, and congestive heart failure) were also recorded. AKI was defined in accordance with KDIGO guidelines24 as an increase in serum creatinine levels exceeding 0.3 mg/dL greater than baseline. Among the patients who survived post-hospitalization, the mortality was analyzed within 3 years after BMT.
The study was submitted and approved by the Institutional Review Board of “Associação Educacional Nove de Julho” report number CAAE: 79907617.4.0000.5511. Patient demographics are described in absolute and relative frequencies, and for continuous variables as measures of central tendency and dispersion. Outcomes are described as relative frequencies with 95% confidence intervals (CI). The Kaplan-Meier method was used to estimate population survival and the log-rank test to verify group differences.
To identify factors that may be associated
with outcomes, univariate analyses were completed with AKI occurrence as a
dependent variable. Pearson's Chi-square test or Fisher's exact test (when
indicated) were used to identify outcome-associated variables. The Mann-Whitney
test was used to analyze the distribution of renal function markers by group.
The prevalence rates of outcomes at two follow-up time points were compared
with the Newman-Keuls test. All statistical analyses were performed in SPSS
software version 20.0. Type I error probability of <5% was considered
statistically significant.
RESULTS
Upon the Institutional Review Board (IRB) approval, medical records were collected for 256 patients who were submitted to BMT between January 2009 and October 2014. During this period, there were changes in the laboratories accredited to perform serum creatinine analysis in the hospital. In these cases, access to the results of some patients was restricted or lost. For this reason, the analysis of many patients was limited to the period between 2012 and 2014, when laboratory tests were performed by the same laboratory, and after the application of inclusion and exclusion criteria, 79 data from patients submitted to BMT between January 2012 and October 2014 were analyzed. The flowchart of the cases included in this study is shown in Figure 1.
|
|
|
Figure 1. Flowchart of patients submitted to BMT included in the study |
This sample consisted predominantly of males (59.5%), with a mean age of 43.1±14.3 years and 13.9% of older patients. The mean body mass index was 26.2±5.5 kg/m2. The diagnoses of NHL (30.4%), acute myeloid leukemia (26.6%) and HL (24.1%) were the most frequent. Comorbidities were infrequent, with obesity and hypertension being the most prevalent, reaching 20.3% and 7.6%, respectively. Just under half of the studied procedures (45.6%) were autologous BMT (Table 1).
The median of the total length of hospital stay was 34.0 (IQR 27.0-48.0) days, with the length of stay of 26.0 (IQR 18.0-40.0) days after BMT, as shown in Table 1.The median length of hospitalization was significantly longer for allogeneic than for autologous transplant patients (39.5 days vs. 25.0 days, p<0.001).
Among the 79 patients analyzed, 54.4% (n=43) had lymphomas, 30.4% (n=24) NHL and 24.1% HL (n=19). For 45.6% of leukemias (n=33), 26.1% were AML (n=21), 13.9%, ALL (n=11) and 3.8% CML (n=3) and 1.3% (n=1) CLL. Demographic and clinical characteristics of the 79 patients are shown in Table 1.
|
Table 1. Demographic and clinical characteristics of the study sample (n=79) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Captions: BMT = bone marrow transplantation; AKI = acute kidney injury; SD = standard deviation. (*) p<0.05. |
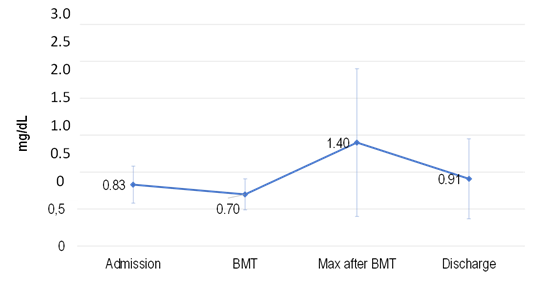
The pattern of serum creatinine levels determined during hospitalization for BMT is shown in Chart 1. AKI occurred in 38.0% of the patients.
|
|
|
Chart 1. Serum creatinine concentration during hospitalization for BMT |
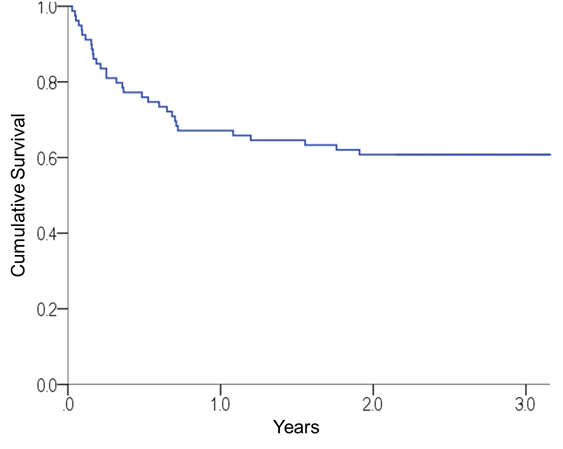
The median time of clinical follow-up among the surviving patients was 2.92 years (range, 2.1-8.0 years). During follow-up, 31 deaths were recorded. The probability of survival 100 days and 3 years after BMT was 81% (95% CI 72.0-89.8%) and 60.8% (95% CI 59.8-71.8%), respectively (Chart 2). After BMT, 1-year, 2-year, and 3-year time points, the number of patients at risk of death were 19, 53, 48, and 20 while the number of cumulative events were 0, 26, 31, and 31, respectively.
|
|
|
Chart 2. Overall survival curve for the sample after BMT |
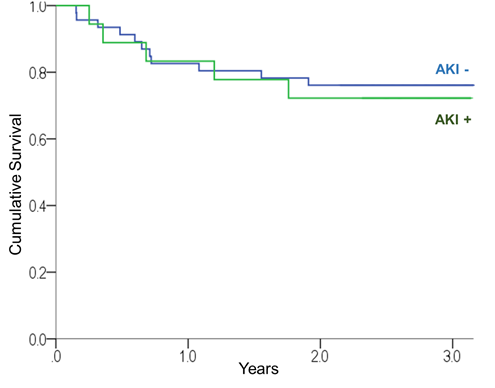
Of the 31 deaths, 14 occurred during post-BMT hospital stay, resulting in an in-hospital mortality rate of 17.7% (95% CI 10.0-27.4%). In-hospital mortality was significantly higher among patients who developed AKI while hospitalized than among those who did not (40.0% vs. 4.2%, chi-square: p<0.001). Among the 64 cases of patients who survived post-hospitalization, there was no significant difference in the overall survival of patients with AKI versus without AKI (log rank: p=0.770, Chart 3).
|
|
|
Chart 3. Survival curves of patient groups with and without AKI during hospitalization |
DISCUSSION
AKI incidence associated with hematopoietic cell transplantation ranges from 10% to 73% depending on the transplantation type and chemotherapeutic conditioning regimen, making it difficult to compare the results of the present study with the literature28. This pathology occurred in 50% of the study sample, slightly less than 60% of the patients with lymphoma who underwent BMT and reported to have developed AKI as concluded by a 2017 US study23. One possible explanation for this difference is the fact that the current sample was extracted from a specialized private hospital in treating malignant neoplasms where only 40% of BMT patients develop AKI22.
A consistent elevation of serum creatinine levels since admission to post-BMT was found in patients who met the AKI diagnosis criteria. The values returned to near baseline upon hospital discharge, indicating that there was no perpetuation of the lesion and there were no cases progressing to CKD in 3 years of follow-up.
Brazil’s Mortality Information System12 reports mortality rate of 14.7% with HL and 36.1% with NHL. In the study sample, the total mortality rate for combined HL and NHL cases was 29.8%4.
This is a retrospective, single-center study and therefore, there are some limitations related to survey biases. In addition, it was not possible to confirm precisely whether the deaths during hospitalization were due to AKI or sepsis; it was not known whether AKI was the direst prognostic factor or if patients developed AKI because they became vulnerable by another, more severe condition. Finally, because the study sample consisted in patients relatively younger (low percentage of patients >60 years old) with few associated comorbidities, it was not possible to analyze the contributions of these factors to the evolution of AKI.
It has been presented a Brazilian epidemiology considering the paucity of studies of this type, and because the existing ones are typically based in data from other countries. It is difficult to compare epidemiological data obtained for the Brazilian population for variables as age, comorbidities, BMI, and socioeconomic profile, with data from INCA because only total data and estimates of cases are published for specific years.
Nevertheless, it was found that obesity, in addition to being associated with a higher incidence of malignant neoplasms is also associated with higher occurrence of AKI29. Conversely, although BMI <18.5 was associated with worse AKI evolution in hospitalized patients previously30, underweight BMI was not investigated as a factor because there were no patients in the study sample.
CONCLUSION
The data presented in this study do not allow to conclude with the required accuracy that there is a strong association between BMT and AKI in these patients, and more prospective studies are needed to confirm whether there is an inherent risk of AKI in patients immediately after BMT. Patients with AKI had significantly higher hospital mortality than those who did not present this pathology. However, the patients who were discharged from the hospital had a 3-year cumulative survival similar to those without AKI. With this, physicians' attention should be redoubled in patients with lymphoma or leukemia submitted to BMT who develop AKI during hospitalization.
ACKNOWLEDGEMENTS
To the Graduation Committee of the A. C. Camargo Cancer Center.
CONTRIBUTIONS
Anna Carolina Mota, Joubert Araujo Alves, Gabriel Stecca Canicoba, Garles Miller Matias Vieira contributed to the study design, acquisition, analysis and interpretation of the data; Germana Alves de Brito contributed to the wording, critical review; Benedito Jorge Pereira contributed to the study design, acquisition, analysis and interpretation of the data, wording and critical review and approved the final version; Aline Baptista, Luis André Silvestre Andrade and Marina Harume Imanishe contributed to the approval of the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
None.
1. Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375(21):2023-36. doi: https://doi.org/10.1056/NEJMoa1605949
2. Appelbaum FR. Consolidation chemotherapy prior to hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Best Pract Res Clin Haematol. 2016;29(4):365-71. doi: https://doi.org/10.1016/j.beha.2016.10.012
3. Silva DB, Pires MM, Nassar SM. Câncer pediátrico: análise de um registro hospitalar. J Pediatr (Rio J). 2002;78(5):409-14. doi: https://doi.org/10.1590/S0021-75572002000500012
4. Instituto Nacional de Câncer. Estimativa 2023: incidência de câncer no Brasil [Internet]. Rio de Janeiro: INCA; 2022 [acesso 2023 fev 27]. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2023.pdf
5. Loh JW, Khiabanian H. Leukemia’s clonal evolution in development, progression, and relapse. Curr Stem Cell Rep. 2019;5:73-81. doi: https://doi.org/10.1007/s40778-019-00157-y
6. Press RD, Eickelberg G, Froman A, et al. Next-generation sequencing-defined minimal residual disease before stem cell transplantation predicts acute myeloid leukemia relapse. Am J Hematol. 2019;94(8):902-12. doi: https://doi.org/10.1002/ajh.25514
7. Xia D, Nardi V, Hasserjian RP. Molecular genetic testing in the diagnosis of myeloid neoplasms. Diagn Histopathol. 2019;25(6):249-59. doi: https://doi.org/10.1016/j.mpdhp.2019.04.004
8. Harris NL, Jaffe ES, Diebold J, et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. J Clin Oncol. 1999;17(12):3835-49. doi: https://doi.org/10.1200/JCO.1999.17.12.3835
9. Araújo LHL, Victorino AP, Melo AC, et al. Linfoma não-Hodgkin de alto grau - revisão de literatura. Rev Bras Cancerol. 2008;54(2):175-83. doi: https://doi.org/10.32635/2176-9745.RBC.2008v54n2.1747
10. Fisher RI, Mauch PM, Harris NL, et al. No Hodgkin’s lymphoma. In: DeVita VT Jr, Hellman S, Rosenberg SA. Cancer: principles and practice of oncology. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. p. 1957-7.
11. Cabanillas F, Velasquez WS, Hagemeister FB, et al. Clinical, biologic, and histologic features of late relapses in diffuse large cell lymphoma. Blood. 1992;79(4):1024-8. doi: https://doi.org/10.1182/blood.V79.4.1024.1024
12. SIM: Sistema de Informação sobre Mortalidade [Internet]. Versão 3.2.1.2. Brasília (DF): DATASUS. [data desconhecida] - [acesso 2023 mar 11]. Disponível em: http://sim.saude.gov.br/default.asp
13. Fedele R, Martino M, Recchia AG, et al. Clinical options in relapsed or refractory Hodgkin lymphoma: an updated review. J Immunol Res. 2015:2015:968212. doi: https://doi.org/10.1155/2015/968212
14. National Cancer Institute (US) [Internet]. Bethesda (MD): National Cancer Institute (US); [date unknown]. Adult Non-Hodgkin Lymphoma Treatment (PDQ®): health professional version; [updated 2023 Feb 17; cited 2013, March 11]. Available from: https://www.cancer.gov/types/lymphoma/hp/adult-nhl-treatment-pdq
15. El-Asmar J, Gonzalez R, Bookout R, et al. Clotrimazole troches induce supratherapeutic blood levels of sirolimus and tacrolimus in an allogeneic hematopoietic cell-transplant recipient resulting in acute kidney injury. Hematol Oncol Stem Cell Ther. 2016;9(4):157-61. doi: https://doi.org/10.1016/j.hemonc.2015.11.001
16. Hingorani S. Renal complications of hematopoietic-cell transplantation. N Engl J Med. 2016;374(23):2256-67. doi: https://doi.org/10.1056/NEJMra1404711
17. Yu ZP, Ding JH, Chen BA, et al. Risk factors for acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Chin J Cancer. 2010;29(11):946-51. doi: https://doi.org/10.5732/cjc.010.10293
18. Parikh CR, McSweeney PA, Korular D, et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62(2):566-73. doi: https://doi.org/10.1046/j.1523-1755.2002.00455.x
19. Hingorani SR, Guthrie K, Batchelder A, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int. 2005;67(1):272-7. doi: https://doi.org/10.1111/j.1523-1755.2005.00078.x
20. Parikh CR, Coca SG. Acute renal failure in hematopoietic cell transplantation. Kidney Int. 2006;69(3):430-5. doi: https://doi.org/10.1038/sj.ki.5000055
21. Santos JCO, Mendonça MAO. Fatores predisponentes para lesão renal aguda em pacientes em estado crítico: revisão integrativa. Rev Soc Bras Clin Med [Internet]. 2015 [acesso 2023 mar 11];13(1):69-74. Disponível em: http://files.bvs.br/upload/S/1679-1010/2015/v13n1/a4780.pdf
22. Troxell ML, Higgins JP, Kambham N. Renal pathology associated with hematopoietic stem cell transplantation. Adv Anat Pathol. 2014;21(5):330-40. doi: https://doi.org/10.1097/PAP.0000000000000034
23. Rosner MT, Perazella MA. Acute kidney injury in patients with cancer. N Engl J Med. 2017;376(18):1770-81. doi: https://doi.org/10.1056/NEJMra1613984
24. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl [Internet]. 2012 [cited 2022 Dec 22];2:1-138. Available from: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf
25. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720-48. doi: https://doi.org/10.1038/s41375-022-01620-2
26. Cree IA. The WHO classification of haematolymphoid tumours. Leukemia. 2022;36:1701-2. doi: https://doi.org/10.1038/s41375-022-01625-x
27. Troxell ML, Higgins JP, Kambham N. Antineoplastic treatment and renal injury: an update on renal pathology due to cytotoxic and targeted therapies. Adv Anat Pathol. 2016;23(5):310-29. doi: https://doi.org/10.1097/PAP.0000000000000122
28. Parikh CR, Yarlagadda SG, Storer B, et al. Impact of acute kidney injury on long-term mortality after nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(3):309-15. doi: https://doi.org/10.1016/j.bbmt.2007.12.492
29. Rosen DC, Kannappan M, Kim Y, et al. The impact of obesity in patients undergoing robotic partial nephrectomy. J Endourol. 2019;33(6):431-7. doi: https://doi.org/10.1089/end.2019.0018
30. Liu AYL, Wang J, Nikam M, et al. Low, rather than high, body mass index is a risk factor for acute kidney injury in multiethnic asian patients: a retrospective observational study. Int J Nephrol. 2018;2018:3284612. doi: https://doi.org/10.1155/2018/3284612
Recebido em 18/10/2022
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde