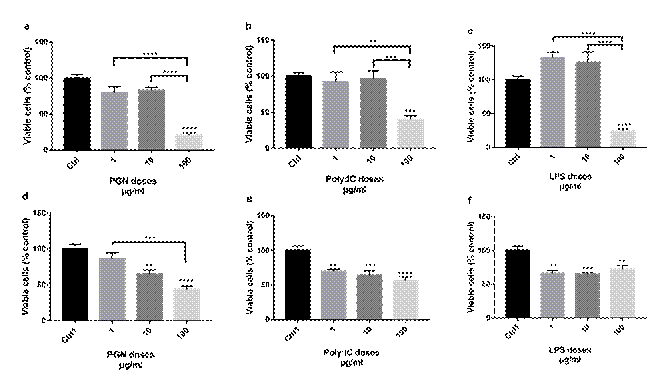ORIGINAL ARTICLE
Toll-like Receptors 2, 3, and 4 in Childhood Acute Lymphocytic Leukemia
Receptores do Tipo Toll 2, 3 e 4 em Leucemia Linfocítica Aguda Infantil
Receptores Tipo Toll 2, 3 y 4 en la Leucemia Linfocítica Aguda Infantil
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n3.3676
Matheus Loureiro da Silva Cruz1; Rafael Pereira dos Santos2; Barbara Kunzler Souza3; Mariane da Cunha Jaeger4; Camila Alves da Silva5; Lauro José Gregianin6; Jiseh Fagundes Loss7; Rebeca Ferreira Marques8; Algemir Lunardi Brunetto9; André Tesainer Brunetto10; Rafael Roesler11; Caroline Brunetto de Farias12
1-12Universidade Federal do Rio Grande do Sul (UFRGS). Porto Alegre (RS), Brazil.
4,9,10,12Instituto do Câncer Infantil. Porto Alegre (RS), Brazil.
1-12E-mails: mathloureiro@gmail.com; rafa.rpds@gmail.com; barbarakunzler@yahoo.com.br; labpesquisa1@ici.ong; eng.camilaalves@gmail.com; lgregianin@hcpa.edu.br; jiseh1992@gmail.com; rfmarques@hcpa.edu.br; institucional@ici.ong; andrebrunetto@ici.ong; rafaelroesler@hcpa.edu.br; carolbfarias@gmail.com. Orcid iD: https://orcid.org/0000-0002-1141-0254; Orcid iD: https://orcid.org/0000-0003-0949-9648; Orcid iD: https://orcid.org/0000-0002-1694-1337; Orcid iD: https://orcid.org/0000-0002-3465-4083; Orcid iD: https://orcid.org/0000-0002-4243-7767; Orcid iD: https://orcid.org/0000-0003-0788-7858; Orcid iD: https://orcid.org/0000-0002-0088-514X; Orcid iD: https://orcid.org/0000-0001-6447-1029; Orcid iD: https://orcid.org/0000-0003-0668-6894; Orcid iD: https://orcid.org/0000-0002-7958-1279; Orcid iD: https://orcid.org/0000-0001-6016-2261; Orcid iD: https://orcid.org/0000-0002-6435-6626
Corresponding author: Matheus Loureiro da Silva Cruz. Rua Ramiro Barcelos, 2350 – Santa Cecília. Porto Alegre (RS), Brazil. CEP 90035-003. E-mail: mathloureiro@gmail.com
ABSTRACT
Introduction: Acute lymphocytic leukemia (ALL) is the most common cancer type in children and accounts for 80% of pediatric leukemias. Novel targets are necessary to improve survival rates for refractory and relapsed disease. There is accumulating evidence that Toll-like receptor (TLR) signaling may be associated with outcomes in cancer however little has been described in leukemias. Objective: Analyze the expression and contribution of TLRs to the development of childhood ALL. Method: To evaluate the effect of specific TLR2, TLR3, and TLR4 agonists on the viability and proliferation of childhood ALL cell lines and to analyzed the mRNA expression of these types of TLR in bone marrow blast cells at diagnosis (D0) and induction (D35) in pediatric ALL patients. Results: Treatment with TLR agonists reduced the cell viability of Jurkat and Sup-B15 cell lines. Cell cycle distribution in Jurkat was altered, reducing polyploid cells and increasing sub-G1 phase. Conclusion: It was observed that the cell viability of the cell lines responded with different sensitivities to the agonists. The polyploidy associated with tumor malignancy was reduced, in addition to the increase in the sub-G1 phase indicating an increase in apoptosis. There were differences in TLR expression at D35 between groups at risk of the disease. Patients with high expression of TLR2 and low expression of TLR4 on D35 demonstrated a worse prognosis.
Key words: precursor cell lymphoblastic leukemia-lymphoma; Toll-like receptors; precursor cell lymphoblastic leukemia-lymphoma.
RESUMO
Introdução: A leucemia linfoblástica aguda (LLA) é o tipo de câncer mais comum em crianças e representa 80% das leucemias pediátricas. Novos alvos são necessários para melhorar as taxas de sobrevivência para doença refratária e recidivante. Há evidências acumuladas de que a sinalização de receptores Toll-Like (TLR) pode estar associada a resultados em câncer, embora pouco tenha sido descrito em leucemias. Objetivo: Analisar a expressão e a contribuição dos TLR para o desenvolvimento da LLA infantil. Método: Avaliar o efeito de agonistas específicos de TLR2, TLR3 e TLR4 na viabilidade e proliferação de linhagens celulares de LLA infantil e analisar a expressão do RNAm desses tipos de TLR em células blásticas da medula óssea no diagnóstico (D0) e na indução (D35) em pacientes LLA pediátricos. Resultados: O tratamento com agonistas de TLR reduziu a viabilidade celular das linhagens celulares Jurkat e Sup-B15. A distribuição do ciclo celular em Jurkat foi alterada, reduzindo as células poliploides e aumentando a fase sub-G1. Houve aumento na expressão dos receptores entre D0 e D35 em amostras de pacientes. Conclusão: Observou-se que a viabilidade celular das linhagens celulares respondeu com diferentes sensibilidades aos agonistas. A poliploidia associada à malignidade tumoral foi reduzida, além de o aumento da fase sub-G1 indicar aumento da apoptose. Houve diferenças na expressão de TLR em D35 entre os grupos de risco da doença. Pacientes com alta expressão de TLR2 e baixa expressão de TLR4 no D35 demonstraram pior prognóstico.
Palavras-chave: leucemia-linfoma linfoblástico de células precursoras; receptores Toll-like; leucemia-linfoma linfoblástico de células precursoras.
RESUMEN
Introducción: La leucemia linfocítica aguda (LLA) es el tipo de cáncer más común en los niños y representa el 80 % de las leucemias pediátricas. Se necesitan nuevos objetivos para mejorar las tasas de supervivencia de la enfermedad refractaria y recidivante. Cada vez hay más pruebas de que la señalización del receptor Toll-Like (TLR) puede estar asociada con resultados en el cáncer, aunque se ha descrito poco en las leucemias. Objetivo: Analizar la expresión y la contribución de los TLR al desarrollo de la LLA infantil. Método: Evaluar el efecto de agonistas específicos de TLR2, TLR3 y TLR4 en la viabilidad y proliferación de líneas celulares de LLA infantil y analizar la expresión de ARNm de estos tipos de TLR en células blásticas de médula ósea en el momento del diagnóstico (D0) y la inducción (D35) en pacientes pediátricos con LLA. Resultados: El tratamiento con agonistas de TLR redujo la viabilidad celular de las líneas celulares Jurkat y sup-B15. Se alteró la distribución del ciclo celular en Jurkat, reduciendo las células poliploides y aumentando la fase sub-G1. Hubo un aumento en la expresión de los receptores entre D0 y D35 en muestras de pacientes. Conclusión: Se observó que la viabilidad celular de las líneas celulares respondía con distintas sensibilidades a los agonistas. Se redujo la poliploidía asociada con la malignidad del tumor, además de un aumento de la fase sub-G1 que indica un aumento de la apoptosis. Hubo diferencias en la expresión de TLR en D35 entre los grupos de riesgo de enfermedad. Los pacientes con alta expresión de TLR2 y baja expresión de TLR4 en D35 mostraron peor pronóstico.
Palabras clave: leucemia-linfoma linfoblástico de células precursoras; receptores Toll-like; leucemia-linfoma linfoblástico de células precursoras.
INTRODUCTION
Currently, the occurrence of cancer in children and teenagers is rare, about 1-4% of all cancers in the general population1, although the overall incidence of childhood cancer, including acute leukemia, has gradually increased since the 1970s2. Acute leukemia is the most common form of pediatric cancer, accounting for nearly a third of cases in individuals younger than 15 years2. Acute lymphocytic leukemia (ALL) is a clonal disease of hematopoietic tissue characterized by the malignant transformation of lymphoid precursor cells into the bone marrow. The incidence increased between 1975 and 2010, with 25 to 34 cases per million, an average increase of 0.7%2. The development of ALL is associated with malignant-like cells that express low levels of immunogenic surface molecules and are poor B and T-cell stimulators, which facilitates their escape from antineoplastic immune responses3-5.
Toll-like receptors (TLRs) are transmembrane glycoprotein receptors belonging to a family of evolutionarily conserved receptors responsible for recognizing a diversity of distinct exogenous and endogenous molecular structures6. TLRs are widely distributed and expressed in many cell types, but mainly in immune cells7. The relation of TLRs to their gene expression, their association with prognostic factors and risk factors, as well as their ligands and their activation in some malignancies have been studied8.
The TLR signaling seems to modulate the transformation of tissue or cells that have undergone a process of chronic inflammation and/or acute premalignant lesions9. Many clinical trials show the relationship between the overexpression of several types of TLRs to a worse prognosis in different neoplasms10. Though, there is scarce knowledge about its role in pediatric leukemias, especially ALL. One of the first studies in leukemia showed that precursor B-cell acute lymphoblastic leukemia (CBP-ALL) expresses TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, and TLR9. However, it was unclear whether the high expression observed from TLR3, TLR4, and TLR5 occurs as a consequence of tumor malignancy or represents a phenotype11,12.
This study aimed to evaluate the expression of TLR2, TLR3, and TLR4 in bone marrow blasts cells from children with acute lymphoblastic leukemia and the role of their specific agonists on cell proliferation and viability as possible mechanisms of cellular signaling that act on these processes in cell lines. Additionally, to correlate the results obtained on the expression of these types of TLR with the outcome and clinical data of the patients.
METHOD
Cell viability and proliferation were evaluated after 48 hours using the following compounds: peptidoglycan (PGN, TLR2 agonist, Sigma # 77140), polyinosine acid (Poly: IC, TLR3 agonist, Sigma # P9583), and lipopolysaccharide (LPS, TLR4 agonist, Sigma # L6529). The concentrations in (μg/ml) were determined based on data from the literature.
The Jurkat and Sup-B15 cell lines were obtained from the American Type Culture Collection (ATCC). Cells were grown in 25 cm2 sterile flasks in RPMI 1640 culture medium (Gibco), supplemented with fetal bovine serum, antibiotic and antifungal. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2. The experiments were executed in at least triplicate. Viability and cell proliferation were performed by the blue-count counting method of Tripan. Briefly, an aliquot of 10 μL of each sample was homogenized in a 10 μl aliquot of 0.4% Trypan blue, and the cell count was carried out on a hemocytometer. Cells dead or damaged in the cell membrane are permeable by the dye. Cell viability is the ratio between stained (non-viable) and non-stained (viable) cells.
To evaluate the cell cycle, the Jurkat cell line was cultured in 24 well plates at a density of 1.5x105 cells/well and treated with PGN, POLY: IC, and LPS at the concentrations of 100 μg/ml, 100 μg/ml, and 50 μg/ml, respectively, and compared to the untreated control. After 24 hours, cells were collected, centrifuged, and washed with PBS twice. The cells were then resuspended in 50 μg/ml propidium iodide (Sigma-Aldrich) in 0.1% Triton X-100 in 0.1% sodium citrate containing 5 μg/ml of RNase A. The cells were stained with 1 ml of propidium iodide (PI) cell cycle solution for 5 minutes, followed by evaluation on the Attune Acoustic flow cytometer (Applied Biosystems, Thermo Fisher Scientific, USA). In each sample, 20,000 cells were analyzed. Data were analyzed using FlowJo Cytometric 10.1 software. Three individual experiments were performed in triplicate.
The sample consisted of 26 pediatric patients (from zero to 18 years old) diagnosed with ALL in the Pediatric Oncology Service of “Hospital de Clínicas de Porto Alegre”, by signing the Informed Consent Form. A volume of 4ml was collected from the bone marrow in vacuum tubes containing EDTA from patients only when requested for clinical evaluation. These evaluations occurred at the time of diagnosis (before treatment started, day zero D0), and at the end of the induction treatment, at day 35 (D35). These experiments were performed in triplicate. The Institutional Review Board (IRB) of “Hospital de Clínicas de Porto Alegre”, CAAE 46929015.7.0000.5327 (Submission for Ethical Review) approved the study (report number 2015-0318) in compliance with Resolution 466/1213 of the National Health Council for research with human beings.
Total RNA extractions from bone marrow samples were performed using the PureLink® RNA Mini Kit (Life Technologies # 12183018A), and the total RNA extraction from the cell lines was carried out by the Trizol method (Invitrogen), both methods according to the manufacturer's instructions. Samples were quantified in Nanodrop® following both quantity and purity aspects.
The mRNA was amplified using the primers of β-actin, TLR2, TLR3, and TLR4 (all sequences were designed according to Gen Bank). Β-actin expression was utilized as control. All samples consisted of a final total volume of 15 μL using 35 cycles for amplification consisting of 1 minute at 95°C, denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension of the primers at 72°C for 45 seconds, followed by a final extension at 72°C for 10 minutes. Electrophoresis was performed in 1% agarose gel (Biotium) of the amplified products and visualized in ultraviolet light. Fragments were confirmed using Low DNA Mass Ladder (Invitrogen), and the relative expression of each primer was determined by densitometry using ImageJ 1.45 software.
The results of the experiments with patients were expressed as mean ± standard error and comparisons between groups were conducted by comparing the average Wilcoxon T-test. Experiments with cell lines were performed separately and independently (n=3). The results of the experiments with cell lines were expressed as mean ± standard error of mean, and comparisons were performed by analysis of variance (ANOVA) followed by post hoc Tukey's. The overall survival (OS) was determined by the Kaplan-Meier method. The log-rank test was used to compare the curves. p < 0.05 indicates a significant difference. The graphs were performed using GraphPad version 7.0 (GraphPad Software, San Diego, CA, USA).
RESULTS
|
|
|
Figure 1. Cell viability of Jurkat cells after 48h of treatment with TLR agonists. (a) PGN treatment; (b) Treatment with Poly: IC; (c) Treatment with LPS. Cell viability of Sup-B15 cells after 48h of treatment with TLR agonists; (d) PGN treatment; (e) Treatment with Poly: IC; (f) Treatment with LPS. The comparisons were performed by analysis of variance (ANOVA), followed by Tukey's post hoc test. (**) p < 0.005; (***) p < 0.0005; (****) p < 0.0001 |
There was a decrease in the cell viability of Jurkat after the treatment with three agonists of TLR2, TLR3, and TLR4, respectively at the concentration of 100 μg/ml (all with p ≤ 0.0001) of the control. Treatments with PGN and LPS reduced cell viability by over 75%, whereas the POLY: IC agonist reduced viability by approximately 50% (Figure 1a, 1b and 1c). In other concentrations tested (1 μg/ml and 10 μg/ml), there was no significant difference in relation to control.
In the Sup-B15 cell line, after 48 hours of treatment with PGN it was observed a decrease in cell viability compared to the control from the concentration of 10 μg/ml (p = 0.0039). At the concentration of 100 μg/ml, there was a reduction of about 50% in cell viability (p < 0.0001; Figure 1d). In the treatments with POLY: IC and LPS, after 48 hours, a reduction in cell viability was observed in the cell line Sup-B15 compared to control at a concentration of 1 μg/ml (p = 0.0029) and (p = 0.0002) (Figure 1e and 1f).
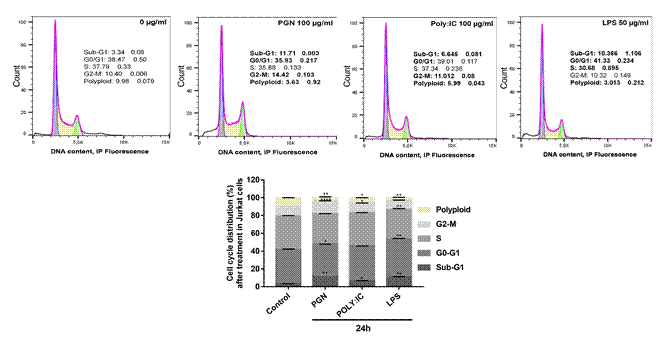
In the cell cycle analysis, after treatment with PGN, POLY: IC, and LPS, respectively, Jurkat cells showed an increase in the sub-G1 phase of the cell cycle (p = 0.0013, p = 0.0412, and p = 0.0026) and a reduction of polyploidy (p = 0.0021, p = 0.012 and p = 0.0015), corresponding p values for each agonist, PGN, POLY:IC and LPS, respectively. In addition, there was an increase in the G2 phase after treatment with PGN and POLY: IC (p < 0.0001 and p = 0.037), and a reduction in the S phase after LPS treatment (p = 0.0016). The G0-G1 phase decreased after treatment with PGN (p = 0.00119) and increased following treatment with LPS (p = 0.0078).
All 26 patients with ALL were distributed in groups according to the disease risk classification, medical criteria and other characteristics (Table 1).
|
Table 1. Demographic data of the patients |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
(a) Number of relapses during the medical follow-up period; (b) Risk was stratified by the criteria of the protocol of the Brazilian Group Treatment of Leukemia in Childhood (GBTLI 2009); (c) Calla Antigen or CD10 is the most common hallmark of childhood ALL. Its absence may indicate the poorer prognosis of the disease; (d) Positive minimal residual disease (MRD) is defined as MRD>0.01 in bone marrow immunophenotyping. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
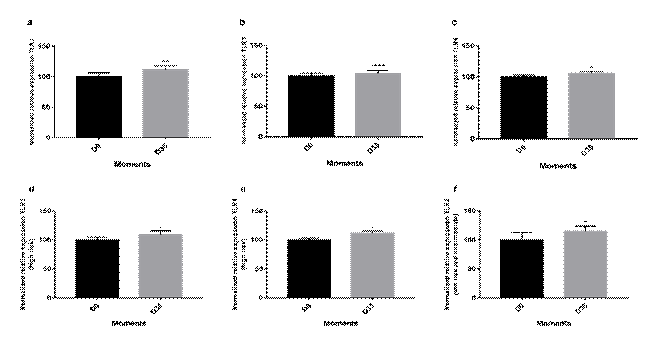
Among the 26 patients, 16 were in the high-risk group, 6, in the intermediate group, and 4 in the low-risk group. In addition to this classification, the patients were separated according to the cell line (B or T). Thus, of the total number of patients, 21 were of B-cell lines and 5 of T-cell lineage patients, of which 4 were in the high-risk group, a pattern corroborated by the literature14. The expression of TLRs in the cells from the bone marrow was analyzed by semiquantitative PCR in D0, as well as in D35. When evaluating the expression of TLR2, TLR3, and TLR4 on values normalized by β-Actin, it was found that there was an increase in the mean and median values of the relative expression of these receptors in D35 comparing with D0. However, the analysis in separate revealed that the relative expressions of these receptors increased at D35 in relation to D0, by 11.58%, 3.89%, and 5.75%, respectively. These differences were considered significant for TLR2 (p = 0.0039), TLR3 (p < 0.0001), and TLR4 (p = 0.0382) (Wilcoxon T-test) (Figure 3a, 3b and 3c).
|
|
|
Figure 3. Relative expression of TLRs in D0 and D35 normalized by β-actin in bone marrow cells from pediatric patients considering all risk stratification groups. (a) Expression of TLR2; (b) Expression of TLR3; (c) Expression of TLR4. From pediatric patients in the high-risk group; (d) Expression of TLR3; (e) Expression of TLR4. From pediatric patients in the low-risk and intermediate groups; (f) Expression of TLR2. The comparisons between the groups performed comparing the means by the Wilcoxon T test. (*) p < 0.05; (**) p < 0.005; (****) p < 0.0001 |
The relative expressions of TLR2, TLR3, and TLR4 receptors in patients in the high-risk group increased in the expression of TLR3 and TLR4 at D35 in relation to D0, respectively, of 9.14% and 11.67% (p = 0.001) and (p = 0.021). These differences were considered significant (Wilcoxon T test) (Figure 3d and 3e). When evaluating the relative expressions of the TLR2, TLR3, and TLR4 receptors in low-risk and intermediate-risk patients, there was an increase in TLR2 expression at D35 compared with D0, of 14.89%. This difference was considered significant (p = 0.048) (Wilcoxon T-test) (Figure 3f).
The median TLR2 expression was 110.76 (Mdn=110.76). The first quartile was 74.72 (Q1=74.72), and the third quartile was 144.43 (Q3=144.43). Patients with the value of TLR2 expression between the median and third quartiles had a lower overall survival (OS) than patients with low expression (p < 0.05). The results are shown in Table 2. The median TLR4 expression was 106.57 (Mdn=106.57). The first quartile was 93.39 (Q1=93.39), and the third quartile was 118.42 (Q3=118.42). Patients with the TLR4 expression value between the median and third quartiles had greater overall survival than patients with low expression (p < 0.05). The results are shown in table 2. There was no significant difference between the overall survival rate and TLR3 expression in D35.
|
Table 2. TLR2 and TLR4 expression and overall survival in patients with ALL |
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
(a) Toll-like receptor 2; (b) Toll-like receptor 4; (c) Overall survival; (d) Standard error of mean; (e) The end of the induction treatment, at day 35; (f) Quartile 1; (g) Median. |
|||||||||||||||||||||||||||||||||||||||||||
DISCUSSION
Tumor cells may develop self-regulation systems and protection mechanisms to overwhelm the immune system15. This anti-immunogenic process is necessary for tumor and immunological microenvironment cancer progression, and understanding the key elements is important for the development of immunotherapy-based therapeutic strategies16.
The stimulation of TLRs plays a crucial role in the homeostasis of mature B lymphocytes17, T lymphocytes, NK cells, monocytes, neutrophils, macrophages, and other cells of the immune system18,19. In some cases, not only the presence of TLRs in cancer signifies a poorer prognosis, but also all the machinery of adapter molecules that participate in the intracellular response of these receptors involved, as well as its extracellular responses20,21.
Among the cellular responses, inflammatory and immunological reactions promote angiogenesis, proliferation, permeability, cellular survival, and pro-apoptotic activity through the production of molecules and cytokines such as TNF-α, TNF-β, VEGF, IL-6, IL-8 (pro-angiogenic and immunogenic chemokine)20,22-24. The overall effect of this activation favors tissue invasion, metastasis formation, and creation of an ideal tumor microenvironment, providing conditions for the advance of the tumor25.
In addition, the inflammatory reaction caused by the TLR-activated pathway is a factor that increases tissue injury, since healthy cells are destroyed and tumor cells appear to be more immune to this process and so they pass the malignant clonal expansion process26,27. A study28 evaluating colorectal cancer (CRC) showed that there is induction of tumor progression from inflammatory processes, changes in the intestinal microbiota, and oncogene activation. Additionally, it was noticed that patients with CRC had higher expression of TLR2 and lower expression of TLR4 than individuals in the control group. They noticed tumor development with tissue changes from upregulation of TLR2 and downregulation of TLR4, showing that lower TLR4 expression was associated with the development of metastases and worse 5-year survival28.
In the present study, the treatment of Jurkat and Sup-B15 cells with the TLR agonists promoted the reduction of cell viability. This effect seems to be dose-dependent, although there were no significant differences in concentrations of 1 μg/ml and 10 μg/ml in the treatment of the Jurkat lineage. In the treatment with PGN, there was no statistical difference from the concentration of 10 μg/ml in the control, and between the control and the concentration of 100 μg/ml. In the treatment with Poly: IC and LPS there was a statistical difference between the concentration of 1 μg/ml and the control, but not among other concentrations.
The difference in response among the treatments can be attributed to the differences between the cell lines utilized. It occurred since agonist sensitivity was lower in Jurkat cells that are derived from T cell ALL, a more aggressive type of leukemia, stratified in the group of high risk. In turn, agonist sensitivity was higher in Sup-B15 cells, which are derived from B-cell ALL, which are stratified as low-risk group.
The study is the first to show differences of chemotherapy-induced expression of TLR2, TLR3, and TLR4 on D35. The analysis of the expression of TLRs in patients between D0 and D35 showed an increased expression of TLR2, TLR3, and TLR4 of 11.58%, 3.89%, and 5.75%, respectively. When analyzing the patients, and separating them by risk groups, the results also revealed that this increase was more significant.
For patients in the high-risk group, the increase in TLR3 and TLR4 expression was 9.14% and 11.67%, respectively. In the analysis of the TLR expression of the patients from the low-risk and intermediate groups, a 15.89% increase in TLR2 expression was noticed. This increase may be associated with the immune system itself, which modulates the proliferation, expansion, and differentiation of immune cells, since in patients in the low-risk and intermediate groups, the increase in TLR2 was more significant. On the other hand, the increase in the expression of TLR3 and TLR4 in patients of the high-risk group can be associated with pro-tumor mechanisms involved in the regulation of mechanisms of differentiation and proliferation of leukemic cells.
The survival analysis of the patients in relation to the expression of TLR2 and TLR4 showed that those with lower overall survival had higher TLR2 expression and lower TLR4 expression in D35. Patients with higher overall survival showed lower expression of TLR2, and TLR4 expression increased. These results concur with other studies that suggest that low TLR4 expression and high expression of TLR2 can be prognostic markers for staging or B-CLL29. Rybka et al. compared healthy subjects to patients with AML (both studies with adults) and showed a relationship between high TLR2 and TLR4 expression with a worse prognosis of the disease. The same group showed a relationship between high TLR2 expression with CLL-staging since TLR2 expression was higher in patients with more advanced stages of AML. Additionally, the reduction of TLR4 expression was associated with more advanced stages and a more unfavorable prognosis30,31.
The first study of childhood ALL showed that the expression of TLR1, TLR3, TLR4, TLR7, and TLR9 was lower between patients and the control group. When TLR expression levels with different ALL subtypes (Pro-B, Pre-B, B, and T) were analyzed, it was found lower TLR1 values for the pre-B and T and TLR4 subtypes for the Pro- B and B. These values were considered extremely lower when compared to the control group32. Cell cycle analysis showed that the agonist-induced treatment increased the cell population in sub-G1, which is indicative of cell death by apoptosis33. On the other hand, the treatment reduced the polyploidy phase, showing that the agonists decreased the proportion of cells with the chromosome increase. Polyploidy is closely associated with genomic instability, tumor heterogeneity induction, and malignancy processes, and characteristics that lead to the inactivation of tumor suppressor genes or oncogenes activation34,35. Treatment with PGN and POLY: IC increased the G2 phase, suggesting that pathways related to protein synthesis are also activated. Treatment with LPS reduced the S phase by about 20%, but increased the G0-G1 phase, suggesting an extension of this to the detriment of the S. Treatment with PGN reduced the G0-G1 phase. Based on cell cycle analysis, it is possible to suggest that the reduction of cell viability observed in Jurkat cells after treatment with specific agonists may be attributed to the activation of pro-apoptotic mechanisms.
Wang et al.35 noticed that treatment with PGN induced TNF-α-dependent apoptosis in cell line acute monocytic leukemia THP-1 and significantly increased gene production of cytokines such as IL-1β, IL-8, and TNF-α and induced phosphorylation of p38, Erk and NF-ĸB. Salaun B et al.36 were the first to show a possible mechanism activated by TLR3 agonists in tumor cells, which identified that this response appears to require IFN-136 and activation of caspase-8 and FADD. As for TLR4, the cascade initiated by agonists has as its mechanism the activation of the apoptotic pathway dependent on caspase-8 and can be attributed to increased production of IFN-α, TNF-α, and down-regulation of NF-ķB37,38. It is still not fully understood by which mechanisms TLRs promote cell growth, cell differentiation, and cell death. Furthermore, it is unclear whether there is a difference in how TLRs influence these mechanisms in normal and tumor cells. Moreover, knowledge about the TLRs has gained importance now for their association with prognoses and outcomes, as well as for the effects caused by agonists or antagonists in cellular pathways that modulate the development and promotion of malignant neoplasms.
CONCLUSION
Treatment with TLR2, TLR3, and TLR4 agonists reduced the cell viability of Jurkat and Sup-B15 cell lines, increased the proportion of cells in sub-G1, which suggests the activation of apoptosis and a reduction in the proportion of cells polyploids. It was also concluded that patients with ALL had increased chemotherapy-induced expression of TLR2, TLR3, and TLR4; in addition to the relationship between the expression of TLR3 and TLR4 and the high-risk group where this increase was greater as well as the possible relationship of TLR2 and the low and intermediate-risk groups, where the increase in expression was significant. After chemotherapy induction, lower overall survival was associated with higher TLR2 and lower TLR4 expression. Other studies with larger samples are necessary to evaluate other moments of the treatment.
CONTRIBUTIONS
The authors contributed to the study design, data analysis, experiments, wording, review and collection of bone marrow samples. They approved the final version for publication.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
FUNDING SOURCES
Ministry of Health/CNPq/FAPERGS PPSUS Process number 1245-2551/13-0. Childhood Cancer Institute (CCI) and the institutional research fund of Hospital de Clínicas de Porto Alegre (FIPE/HCPA 2015-0318). Porto Alegre (RS), Brasil.
REFERENCES
1. Instituto Nacional de Câncer. Estimativa 2023: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2022 [acesso 2023 jul 3]. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2023.pdf
2. National Cancer Institute (US) [Internet]. Bethesda (MD): NIH; [2023]. Childhood acute lymphoblastic leukemia treatment (PDQ®) - Health professional version; 2023 Apr 11 [cited 2022 Jun 8]. Available from: https://www.cancer.gov/types/leukemia/hp/child-all-treatment-pdq
3. Wells G, Kennedy PT, Dahal LN. Investigating the role of indoleamine 2,3-dioxygenase in acute myeloid leukemia: a systematic review. Front Immunol. 2021;12:651687. doi: https://doi.org/10.3389/fimmu.2021.651687
4. Rabe JL, Gardner L, Hunter R, et al. IL12 abrogates calcineurin-dependent immune evasion during leukemia progression. Cancer Res. 2019;79(14):3702-13. doi: https://doi.org/10.1158/0008-5472.CAN-18-3800
5. Arandi N, Ramzi M, Safaei F, et al. Overexpression of indoleamine 2,3-dioxygenase correlates with regulatory T cell phenotype in acute myeloid leukemia patients with normal karyotype. Blood Res. 2018;53(4):294-8. doi: https://doi.org/10.5045/br.2018.53.4.294
6. Favere K, Bosman M, Delputte PL, et al. A systematic literature review on the effects of exercise on human Toll-like receptor expression. Exerc Immunol Rev [Internet]. 2021 [cited 2022 Jun 10];27:84-124. Available from: https://img1.wsimg.com/blobby/go/2b32b969-f24b-4b6f-8e61-5d111c218d54/EIR%202021%20Komplett%20KO3.pdf
7. Sahoo BR. Structure of fish Toll-Like Receptors (TLR) and NOD-like receptors (NLR). Int J Biol Macromol. 2020;161:1602-17. doi: https://doi.org/10.1016/j.ijbiomac.2020.07.293
8. Ayala-Cuellar AP, Cho J, Choi KC. Toll-like receptors: a pathway alluding to cancer control. J Cell Physiol. 2019;234(12):21707-15. doi: https://doi.org/10.1002/jcp.28879
9. Sfanos KS. Targeting Toll-like receptors in cancer prevention. Cancer Prev Res (Phila). 2018;11(5):251-4. doi: https://doi.org/10.1158/1940-6207.CAPR-18-0079
10. Li TT, Ogino S, Qian ZR. Toll-like receptor signaling in colorectal cancer: carcinogenesis to cancer therapy. World J Gastroenterol. 2014;20(47):17699-708. doi: https://doi.org/10.3748/wjg.v20.i47.17699
11. Iacobucci I, Papayannidis C, Lonetti A, et al. Cytogenetic and molecular predictors of outcome in acute lymphocytic leukemia: recent developments. Curr Hemat Malig Rep. 2012;7(2):133-43. doi: https://doi.org/10.1007/s11899-012-0122-5
12. Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, et al. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112(6):2205-13. doi: https://doi.org/10.1182/blood-2008-02-140673
13. Conselho Nacional de Saúde (BR). Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília, DF. 2013 jun 13; Seção 1:59.
14. Maino E, Scattolin AM, Viero P, et al. Modern immunotherapy of adult B-lineage acute lymphoblastic leukemia with monoclonal antibodies and chimeric antigen receptor modified t cells. Mediterr J Hematol Infect Dis. 2015;7(1):e2015001. doi: https://doi.org/10.4084/MJHID.2015.001
15. Campesato LF, Silva APM, Cordeiro L, et al. High IL-1R8 expression in breast tumors promotes tumor growth and contributes to impaired antitumor immunity. Oncotarget. 2017;8(30):49470-83. doi: https://doi.org/10.18632/oncotarget.17713
16. Zou W. Mechanistic insights into cancer immunity and immunotherapy. Cell Mol Immunol. 2018;15(5):419-20. doi: https://doi.org/10.1038/s41423-018-0011-5
17. Chen Z, Wang JH. How the signaling crosstalk of B Cell Receptor (BCR) and Co-receptors regulates antibody class switch recombination: a new perspective of checkpoints of BCR signaling. Front Immunol. 2021;12:663443. doi: https://doi.org/10.3389/fimmu.2021.663443
18. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373-84. doi: https://doi.org/10.1038/ni.1863
19. Sivori S, Pende D, Quatrini L, et al. NK cells and ILCs in tumor immunotherapy. Mol Aspects Med. 2021;80:100870. doi: https://doi.org/10.1016/j.mam.2020.100870
20. Collinson-Pautz MR, Chang WC, Lu A, et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leuk. 2019;33:2195-2207. doi: https://doi.org/10.1038/s41375-019-0417-9
21. Szymańska A, Bojarska-Junak A, Drobiecki A, et al. TLR2 expression on leukemic B cells from patients with chronic lymphocytic leukemia. Arch Immunol Ther Exp (Warsz). 2019;67(1):55-65. doi: https://doi.org/10.1007/s00005-018-0523-9
22. Collins PE, Somma D, Kerrigan D, et al. The IκB-protein BCL-3 controls Toll-like receptor-induced MAPK activity by promoting TPL-2 degradation in the nucleus. Proc Natl Acad Sci USA. 2019;116(51):25828-38. doi: https://doi.org/10.1073/pnas.1900408116
23. Spaner DE, Venema R, Huang J, et al. Association of blood IgG with tumor necrosis factor-alpha and clinical course of chronic lymphocytic leukemia. EBioMedicine. 2018;35:222-32. doi: https://doi.org/10.1016/j.ebiom.2018.08.045
24. Luo X, Zhang X, Gan L, et al. The outer membrane protein Tp92 of Treponema pallidum induces human mononuclear cell death and IL-8 secretion. J Cell Mol Med. 2018;22(12):6039-54. doi: https://doi.org/10.1111/jcmm.13879
25. Khajeh Alizadeh Attar M, Anwar MA, Eskian M, et al. Basic understanding and therapeutic approaches to target toll-like receptors in cancerous microenvironment and metastasis. Med Res Rev. 2018;38(5):1469-84. doi: https://doi.org/10.1002/med.21480
26. Meliț LE, Mărginean CO, Mărginean CD, et al. The relationship between Toll-like receptors and Helicobacter pylori-related gastropathies: still a controversial topic. J Immunol Res. 2019;2019:8197048. doi: https://doi.org/10.1155/2019/8197048
27. Spanou E, Kalisperati P, Pateras IS, et al. Genetic Variability as a regulator of TLR4 and NOD signaling in response to bacterial driven DNA Damage Response (DDR) and inflammation: focus on the gastrointestinal (GI) tract. Front Genet. 2017;8:65. doi: https://doi.org/10.3389/fgene.2017.00065
28. Paarnio K, Väyrynen S, Klintrup K, et al. Divergent expression of bacterial wall sensing Toll-like receptors 2 and 4 in colorectal cancer. World J Gastroenterol. 2017;23(26):4831-8. doi: https://doi.org/10.3748/wjg.v23.i26.4831
29. Rybka J, Butrym A, Wróbel T, et al. The expression of Toll-like receptors in patients with B-cell chronic lymphocytic leukemia. Arch Immunol Ther Exp (Warsz). 2016;64(Suppl 1):147-50. doi: https://doi.org/10.1007/s00005-016-0433-7
30. Rybka J, Butrym A, Wróbel T, et al. The expression of Toll-like receptors in patients with acute myeloid leukemia treated with induction chemotherapy. Leuk Res. 2015;39(3):318-22. doi: https://doi.org/10.1016/j.leukres.2015.01.002
31. Sánchez-Cuaxospa M, Contreras-Ramos A, Pérez-Figueroa E, et al. Low expression of Toll-like receptors in peripheral blood mononuclear cells of pediatric patients with acute lymphoblastic leukemia. Int J Oncol. 2016;49(2):675-81. doi: https://doi.org/10.3892/ijo.2016.3569
32. Maharjan S, Park BK, Lee SI, et al. Gomisin G suppresses the growth of colon cancer cells by attenuation of AKT phosphorylation and arrest of cell cycle progression. Biomol Ther (Seoul). 2019;27(2):210-5. doi: https://doi.org/10.4062/biomolther.2018.054
33. Alonso-Lecue P, Pedro I, Coulon V, et al. Inefficient differentiation response to cell cycle stress leads to genomic instability and malignant progression of squamous carcinoma cells. Cell Death Dis. 2017;8:e2901. doi: https://doi.org/10.1038/cddis.2017.259
34. Jo Y, Shin DY. Repression of the F-box protein Skp2 is essential for actin damage-induced tetraploid G1 arrest. BMB Rep. 2017;50(7):379-83. doi: https://doi.org/10.5483/bmbrep.2017.50.7.063
35.Wang D, Xiao PL, Duan HX, et al. Peptidoglycans promotes human leukemic THP-1 cell apoptosis and differentiation. Asian Pac J Cancer Prev. 2012;13(12):6409-13. doi: https://doi.org/10.7314/apjcp.2012.13.12.6409
36. Salaun B, Zitvogel L, Asselin-Paturel C, et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res. 2011;71(5):1607-14. doi: https://doi.org/10.1158/0008-5472.CAN-10-3490
37. Li H, Gao C, Liu C, et al. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed Pharmacother. 2021;137:111332. doi: https://doi.org/10.1016/j.biopha.2021.111332
38. Gliozzi M, Scicchitano M, Bosco F, et al. Modulation of nitric oxide synthases by oxidized ldls: role in vascular inflammation and atherosclerosis development. Int J Mol Sci. 2019;20(13):3294. doi: https://doi.org/10.3390/ijms20133294
Recebido em 14/3/2023
Aprovado em 12/6/2023
Associate-Editor: Claudio Gustavo Stefanoff. Orcid iD: https://orcid.org/0000-0001-7050-3269
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde