ORIGINAL ARTICLE
Metastatic Castration-Resistant Prostate Cancer in Brazil: a Real-World Investigation Using INCA Database
O Câncer de Próstata Metastático Resistente à Castração no Brasil: um Estudo do Mundo Real Usando o Banco de Dados do INCA
El Cáncer de Próstata Metastático Resistente a Castración en el Brasil: Investigación en el Mundo Real Usando la Base de Datos del INCA
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n2.3763
Lorena Pozzo1; Mércia Liane de Oliveira2; Lucilena Rebelo Monteiro3; Mario Olímpio de Menezes4; Francesco Giammarile5; Marcus Vinícius Sadi6
1,3,4Instituto de Pesquisas Energéticas e Nucleares. São Paulo (SP), Brazil. E-mails: lorena.pozzo@ipen.br; luciremo@gmail.com; mario@ipen.br. Orcid iD: https://orcid.org/0000-0002-3850-5819; Orcid iD: https://orcid.org/0000-0002-4457-4925; Orcid iD: https://orcid.org/0000-0003-0263-3541
2Centro de Tecnologias Estratégicas do Nordeste. Recife (PE), Brazil. E-mail: mercialoliveira@gmail.com. Orcid iD: https://orcid.org/0000-0001-6410-4157
5Human Health Department, International Atomic Energy Agency. Vienna, Austria. E-mail: f.giammarile@iaea.org. Orcid iD: https://orcid.org/0000-0002-0550-7893
6Universidade Federal de São Paulo. São Paulo (SP), Brazil. E-mail: mvsadi@gmail.com. Orcid iD: https://orcid.org/0000-0001-8332-9343
Corresponding author: Lorena Pozzo. Instituto de Pesquisas Energéticas e Nucleares. Av. Professor Lineu Prestes, 2242 – Butantã. São Paulo (SP), Brazil. CEP 05508-000. E-mails: lorena.pozzo@ipen.br; lorenaipen@gmail.com
ABSTRACT
Introduction: Prostate cancer is the second most common cancer in men worldwide. In Brazil, regional disparities in incidences of intermediate and high-risk in late-diagnosed PC cases are expected. Objective: To investigate the clinical and demographic profiles of patients treated with metastatic castration-resistant prostate cancer (mCRPC) in Brazil, using real-world data from public databases. Method: Prostate cancer data from the Brazilian cancer registries were filtered from Brazilian public databases from 2008 to 2018. The number of health institutions and registries at a cancer public database were used to separate the Brazilian Federative Units into two comparison groups. mCRPC patients were estimated by using a combination of filters of staging and treatment (Tx, Nx and M1 + chemotherapy). The patients’ median age and the number and type of treatments were evaluated. Results: A total of 325,987 patients with prostate cancer and 5,367 patients with mCRPC were identified. The median age of the mCRPC patients was 63 years. The percentage of patients who underwent one, two or three treatments was 21.0%, 43.2% and 28.1%, respectively. In addition, management differences were noticed depending on the group analyzed. Conclusion: The results revealed regional discrepancies in the distribution of registered mCRPC patients in the Brazilian territory and in their treatment. This information can be used to strengthen the recently updated treatment and improve the palliative care offered. This work presents suggestions to improve specific prostate cancer databases.
Key words: prostatic neoplasms, castration-resistant; neoplasm metastasis; public reporting of healthcare data.
RESUMO
Introdução: O câncer de próstata é o segundo tipo mais comum em homens ao redor do mundo. No Brasil, diferenças regionais de incidência em casos de risco intermediário e alto tardiamente diagnosticados são esperadas. Objetivo: Investigar os perfis clínico e demográfico de pacientes com câncer de próstata metastático resistente à castração (mCRPC) tratados no Brasil usando dados do mundo real de bancos de dados públicos brasileiros. Método: Os casos de câncer de próstata foram filtrados a partir dos registros brasileiros de câncer no período de 2008 a 2018. O número de instituições de saúde que registram esses casos foi usado para separar as Unidades Federativas brasileiras em dois grupos. O número de pacientes com mCRPC foi estimado usando uma combinação de filtros de estadiamento e tratamento (Tx, Nx e M1 + quimioterapia). A idade média e o número e tipos de tratamento realizados foram avaliados. Resultados: O estudo identificou 325.987 pacientes com câncer de próstata e 5.367 com mCRPC. A mediana das idades de pacientes com mCRPC foi de 63 anos. O percentual de pacientes submetidos a um, dois ou três tratamentos foi de 21,0%, 43,2% e 28,1%, respectivamente. Foram observadas diferenças de manejo nos grupos analisados. Conclusão: Os resultados revelaram diferenças regionais nas distribuições de pacientes com mCRPC no território brasileiro e no manejo da doença. Essa informação pode subsidiar decisões de incorporação de novos tratamentos e de melhoria dos cuidados paliativos oferecidos aos pacientes com mCRPC. Este trabalho apresenta sugestões para o desenvolvimento de bancos de dados específicos para câncer de próstata e aprimoramento dos já existentes.
Palavras-chave: neoplasias de próstata resistentes à castração; metástase neoplásica; registros públicos de dados de cuidados de saúde.
RESUMEN
Introducción: El cáncer de próstata es el segundo tipo más común en hombres en el mundo. En el Brasil, se espera encontrar diferencias regionales en la incidencia de diagnósticos tardíos de riesgo intermedio y alto. Objetivo: Investigar los perfiles clínico y demográfico de pacientes con cáncer de próstata metastásico resistente a la castración (mCRPC) tratados en el Brasil utilizando datos del mundo real de bases de datos públicas brasileñas. Método: Los casos de cáncer de próstata fueron filtrados a partir de los registros brasileños de cáncer nel período de 2008 a 2018. El número de instituciones registradoras en la base de datos fue utilizado para separar los Estados Brasileños en dos grupos para comparación. El número de pacientes se estimó mediante una combinación de filtros de estadio y tratamiento (Tx, Nx y M1 + quimioterapia). Fueron evaluados la edad media y la cantidad y tipos de tratamiento realizados. Resultados: Se identificaron un total de 325.987 pacientes con cáncer de próstata y 5367 pacientes con mCRPC. La mediana de la edad de los pacientes con mCRPC fue de 63 años. El porcentaje de pacientes sometidos a uno, dos o tres tratamientos fue del 21,0%, 43,2% y 28,1%. Fueron observadas diferencias de manejo según el grupo analizado. Conclusión: Fueron reveladas diferencias regionales en la distribución de los pacientes con mCRPC en el Brasil y, especialmente, en el manejo de la enfermedad a partir de bases de datos públicas. Esta información puede apoyar las decisiones de incorporar nuevos tratamientos y mejorar los cuidados paliativos ofrecidos a los pacientes. Se presentan sugerencias para el desarrollo de bases de datos específicas y la mejora de las existentes.
Palabras clave: neoplasias de la próstata resistentes a la castración; metástasis de la neoplasia; reportes públicos de datos en atención de salud.
INTRODUCTION
Prostate cancer (PC) is the second most common cancer in men worldwide, with 1,414,259 new cases estimated in 20201 and 71,730 in Brazil2. The heterogeneity of geographical incidence is known and is thought to occur due to differences in genetic susceptibility, availability, and access to medical care, especially regarding the use of prostate-specific antigen (PSA)-based screening3. As pointed out by Bray et al.4, in high or rising Human Development Index (HDI) countries the incidence of some types of specific cancers, including, prostate, increased.
In Brazil, great differences of clinical approach to PC are noticed among regions, with the South and Southeast regions presenting higher numbers of registered PC cases closer to the international levels2. However, other regions may have different incidences of intermediate and high-risk cases of PC diagnosed at a later stage.
Radical prostatectomy (RP), with or without lymphadenectomy, and radiotherapy, external or brachytherapy, are the main curative treatments and are adjusted depending on the risk stratification determined by clinical examination, PSA and Gleason score5. Between 27% and 53% of all patients undergoing RP or RT develop a rising PSA (PSA or biochemical recurrence). The PSA level that defines treatment failure depends on the primary treatment5. When established, biochemical recurrence can be treated with salvage radiotherapy, with or without androgen deprivation therapy (ADT), or only with ADT.
The treatment of choice depends on the initial curative treatment, tumor location and clinical response. The extent of the disease can be assessed using imaging techniques and successive PSA measurements. The standard treatment for patients with metastatic hormone-naïve (sensitive) PC is ADT because testosterone plays an important role in the growth of tumor cells6. After an initial good response to the ADT, patients develop resistance to this therapy and may become candidates for chemotherapy5,7.
Metastatic castration-resistant prostate cancer (mCRPC) is characterized by rising levels of PSA (≥ 2 ng/ml), and/or radiological progression, despite ADT (testosterone levels < 50 ng/dl or < 1.7 nmol/l)8. Under these conditions, the disease is usually lethal9. The most common site of metastasis for PC is bone, occurring in 80% to 90% of men with metastatic prostate cancer10, followed by regional lymph nodes and other rare sites of metastases. Nevertheless, the introduction of new systemic therapies for mCRPC is changing the natural history of the disease. Nafissi et al.11 have found that visceral metastasis increased from 26.1% in 2009 to 40% in 2016.
Although challenging, the management of patients with mCRPC has changed in recent years with the introduction of new therapeutic approaches designed to increase survival and quality-of-life5. These therapeutic options include the use of new androgen receptor inhibitors (such as abiraterone, enzalutamide, apalutamide or darolutamide), targeted therapy (olaparib or rucaparib), chemotherapy (docetaxel or cabazitaxel), and radium-223 (for bone metastasis). More recently, radioligand therapy with beta and alpha emitters has been used when other options fail (PSMA labelled with Lutetium-177 or Actinium-225)12,13.
Brazilian guidelines provided by CONITEC – “Comissão Nacional de Incorporação de Tecnologias no Sistema Único de Saúde” in 201614 suggest hormonal therapy (1st and 2nd lines) followed by chemotherapy, if necessary. The Public Health System – SUS, under specific demands, funds public hospitals or Centers of High Complexity Oncology Care (CACON), Units of High Complexity Oncology Care (UNACON) in addition to other partners. New therapies, such as 177LuPSMA or 223Ra, should be applied to these patients after being submitted to two hormonal therapies and one chemotherapy, but they are not funded by SUS.
In recent years some studies using real-world data (RWD) have tried to assess data about mCRPC patients using local databases15-18. There are no specific data on the incidence of mCRPC in Brazil.
As stated by the U.S. Food & Drugs Administration (FDA)19 in its framework, RWD is data about the patient health status and/or the delivery of health care routinely collected from a variety of sources and real-world evidence (RWE) is the clinical evidence about the usage and potential benefits or risks of a medical product derived from the analysis of RWD.
In Brazil, CACON and UNACON were ruled in 2005 (Ordinance MS number 741 of the Health Attention Secretary)20. Each of them must create and maintain hospital cancer registries in a digital format, as determined by the National Cancer Institute (INCA). Since 2007 INCA is responsible for the Brazilian national base of Hospital Cancer Registries (HBCR). The web-based tool Integrador RHC21 was developed to receive data regularly sent by SUS approved oncology care hospitals on a mandatory base (optional for private hospitals). An increase in local HBCR sending data over the years is expected.
This database includes systematic information obtained from general practitioners and oncology hospitals21. INCA includes data from the diagnosis, treatment, and disease evolution of malignant neoplasms in Brazilian public, private, philanthropic, and university hospitals. Data collection was standardized using national and international codes and classifications. Personal characteristics, clinical data, and disease information were collected.
The Informatics Department of the Brazilian Public Health System (DATASUS) offers different health and economic information databases. Secondary and administrative data are continually recorded in different databases, such as the National Register of Health Establishments (CNES)22. These data were collected by the Health States Secretaries based on different institutional demands.
In the present study, the objective was to obtain clinical and demographic profiles of mCRPC patients treated in Brazil using RWD from public databases. Currently, to the best of the existing knowledge, there is no study on the mCRPC patient distribution in the Brazilian territory. Understanding the evolution of the PC patient population could guide management and health policies and ultimately improve patient outcomes.
METHOD
Retrospective study for the period 2008 to 2018 utilizing deidentified public and available databases from RHC/INCA21. The Institutional Review Board (IRB) review and approval was waived because only deidentified and public database was utilized in compliance with Resolution 510/16 of the National Health Council23. All datasets from Registering Health Establishments (public, private, philanthropic or university health care units) are subject to quality control and reviewed periodically21. The cases are classified as analytic or non-analytic. The first corresponds to those followed up periodically by the registering hospital, which is responsible for treatment planning, the treatment itself, as well as prescription, tests, and results. Otherwise, the cases were classified as non-analytic.
The “Instituto Brasileiro de Geografia e Estatística” (IBGE) estimated the age distribution of the Brazilian male population from 2010 to 2018 based on the 2010 National Demographic Census24. For 2008 and 2009 the authors used the 2010 age distribution, as there were no available data for those years.
From 2008 to 2018, cancer records were extracted, merged, and cleaned using a fully documented and validated data process (©2021 Tableau Software, LLC, a Salesforce Company). All figures were created using the same software. Only the structured data25 were processed according to the following steps:
1. The cleaning data process kept only patient registers with a primary PC diagnosis (ICD=C61) from RHC/INCA database (analytical and non-analytical) by Brazilian Federative Unit and by year, per the male population by Federative Unit according to IBGE data.
2. The number of cases by CNES, available also at the RHC/INCA database, were extracted and divided by Federative Unit male population according to IBGE data.
3. Steps 1 and 2 allowed the classification of PC patient data per Federative Unit and staging (1 up to 4 or NO INFO when staging was unavailable) and calculation of the number of CNES per Federative Unit and per million men.
4. Considering the difference in health care access and registering scenarios in each of the 27 Brazilian Federative Units, direct comparisons would be unfeasible. Two groups were identified instead (Group 1 – G1 and Group 2 – G2) based on similar characteristics (the number of CNES per 1 million men and the registry quantity at RHC/INCA database per 100 thousand men) and used for data analysis.
Initially, according to CONITEC recommendation for PC treatment14, filters were applied to select patients undergoing two hormonal therapies and one chemotherapy to characterize mCRPC patients. Nevertheless, hormonal therapies showed a very low registration level at RHC/INCA database.
Alternatively, as patients with mCRPC should be submitted to palliative chemotherapy, the following combination of filters to characterize these patients was chosen: Tx, Nx and M1 and 1 chemotherapy.
The data were also analyzed considering the groups of Brazilian Federative Units to which that population belonged. The medians of patient ages and the treatments they had access to were obtained. Analytical and non-analytical cases were considered to extract PC cases, staging and geographical localization but only analytical cases were utilized to assess treatment profiles once patient follow up is needed.
RESULTS
Between 2008 and 2018, the Brazilian health establishments associated with INCA registered 325,987 cases of prostate cancer. The main basis for PC diagnosis was the primary tumor histological findings (approximately 15,000 cases per year). Of the total PC patients, 79,252 were classified as non-analytical cases, indicating that their treatment was not completely supervised by the registering CNES22. All Federative Units showed a reduction of the number of registered cases in 2017 and 2018. These data have not been updated until now due to COVID-19 pandemic related delays. During the same period, from a total of 335 RHC in Brazil, only 204 of them which registered PC cases were found at RHC/INCA database.
The PC patients registered at the INCA database were assessed according to Federative Unit and staging, as presented in Table 1. In most Brazilian Federative Units, more than 50% of patients registered had no staging information, while Piauí (PI), São Paulo (SP), Tocantins (TO), Maranhão (MA), Rio Grande do Norte (RN), Minas Gerais (MG), Paraná (PR), Bahia (BA), and Rio Grande do Sul (RS) had less than 50% of "no information". The States with the largest populations (SP and MG) registered more PC cases at this database.
It was hypothesized that the number of PC patients and their staging assessment could be highly dependent on the number and the characteristics of the health units available; therefore, the number of CNES per million men in each Brazilian Federative Unit is also presented in Table 1. A total of 0.8 up to 6.2 health facilities in charge of PC treatment and data registration at INCA were available per million men in Brazilian Federative Units.
|
Table 1. Total prostate cancer cases, percentage of staging by Federative Unit of Brazil between 2008 and 2018 and number of CNES per million men per state |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Source: Integrador RHC21; CNES22. Captions: CNES = National Register of Health Establishment; n = Total Prostate Cancer Cases. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
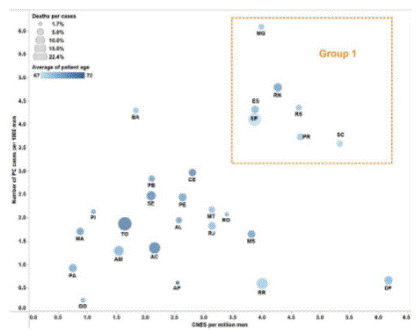
The number of PC cases (per 100,000 men) registered at RHC/INCA21 database was evaluated as a function of the number of CNES per million men (Figure 1). It was possible to identify the distinct groups of Brazilian Federative Units: Group 1 (higher number of cases and higher number of CNES, both per million men), and Group 2. Except for Rio Grande do Norte (RN), all the states of Group 1 are from the South and Southeast regions encompassing states with the highest HDI in Brazil.
Figure 1 also shows the mean age of PC patients at the first diagnosis (color scale) and the percentage of deaths per case for each Brazilian Federative Unit (circle size). Tocantins (TO), Acre (AC), and Amapá (AP) had the highest PC patients mean age (darker circles: 72 to 69), while (TO), São Paulo (SP), Acre (AC), and Roraima (RR) presented the highest mortality rates (22.4%, 21%, 15.9%, and 15.4%, respectively).
|
|
|
Figure 1. Prostate cancer registered cases per 1000 men per number of CNES per million men. The diameter of the circles represents the death rate per case of the disease (ranging from 1.7% to 22.5%); the color indicates the mean age of patients registered at the base (from 67, in lighter blue, to 72 years, in darker blue). All data (analytical and non-analytical) were extracted from RHC/INCA database Source: Integrador RHC21. |
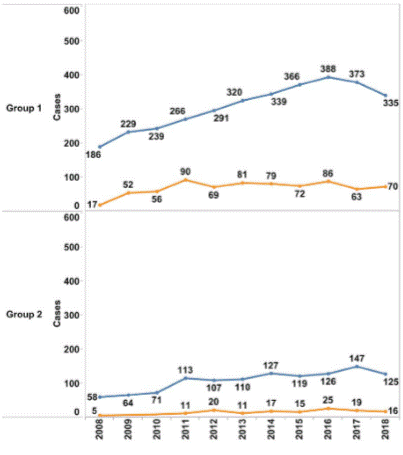
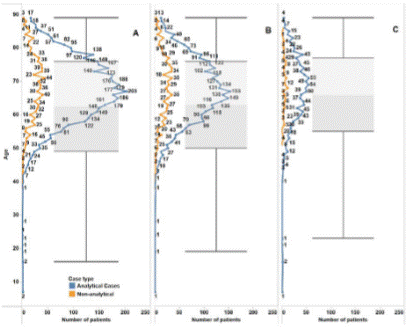
The mCRPC population was estimated as described in Methods. Data analyses were performed by splitting the registered cases into Groups 1 and 2 (Figure 2). Of the 5,367 registries obtained (1.65% of all PC cases), 4,067 were in Group 1 (1.25%) and 1,319 in Group 2 (0.40%). The median age of the patients was 63 years; in Group 1, the median was 63 and in Group 2, 66 years (Figure 3).
|
|
|
Figure 2. mCRPC patients identified per year for Group 1 and Group 2, divided into analytic (blue curve) and non-analytic cases (orange curve) Source: Integrador RHC21. |
|
|
|
Figure 3. Total mCRPC patients estimated (A), for Group 1 (B) and Group 2 (C), by age. The blue curve represents the analytical cases, and the orange curve, the non-analytical. The median ages were calculated considering only the analytical data Source: Integrador RHC21. |
Treatments accessed by mCRPC patients in Groups 1 and 2 are shown in Table 2. 21.00% of the patients received only chemotherapy, while 43.23% received two treatments (chemotherapy and another treatment), 28.08% received three (chemotherapy plus two treatments), and 7.01% accessed four treatments (chemotherapy plus three treatments).
Data suggests that there were no differences between the percentages of patients undergoing only chemotherapy in Group 1 (21.09%) and Group 2 (20.75%), as well as those who received four treatments in Group 1 (6.94%) and Group 2 (7.20%). It appears that in Group 2, a higher number of patients submitted only to two treatments (45.28%) compared to Group 1 (42.51%). However, Group 1 seems to receive one more treatment (28.87%) than Group 2 (25.81%).
Of the patients who received two types of treatment, most of the patients in Group 1 (66.36%) received chemotherapy followed by hormone therapy. On the other hand, in Group 2, 35.04% of the patients underwent hormone therapy, 32.01% radiotherapy, or 30.68% surgery, after chemotherapy.
Considering three treatments, patients of Group 1 received mainly chemotherapy, radiotherapy, and hormonal therapies (52.87%), chemotherapy, hormonal therapies, and surgery (19.60%), or chemotherapy, radiotherapy, and surgery (12.10%). Patients of Group 2 received chemotherapy, hormonal therapy, and surgery (36.54%), chemotherapy, hormonal, and radio therapies (28.57%), or chemotherapy, radiotherapy, and surgery (21.59%).
|
Table 2. Treatments accessed by patients in Groups 1 and 2 (analytical cases only) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Source: Integrador RHC21.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DISCUSSION
About 1.4 million new cases of PC and 375,000 deaths were estimated in 2020 worldwide, being the most diagnosed cancer in men in more than 110 countries, including Brazil1.
Well-established risk factors for PC include age, race, and family history3. Other factors, such as diet and sedentary lifestyle, also seem to contribute to disease development3, but there is a consensus that the high incidence should be attributed to the improvement of the diagnostic procedures and the dissemination of PSA blood tests. In a study published by Teoh et al.26, approximately 59% of men >79 years had PC cells in post mortem studies.
In Brazil, the INCA estimates 71,730 new cases of PC for each year of the triennium 2023-20252. However, cases of PC in Brazil are not uniform throughout the territory. Factors such as access to health services, life expectancy, and ethnicity may influence27-29 and could be related to HDI variations.
Data analysis allowed to identify two groups of very distinct scenarios, Groups 1 and 2. Except for Rio Grande do Norte (RN), Group 1 gathers the Brazilian Federative Units with the highest HDI (0.766)30 and life expectancy (74.3-76.4 years), according to IBGE for 201831.
Although the Federative Units in Group 1 appear to have more cases of PC per thousand men, it is not possible to affirm that this is a real-world scenario. Although a higher HDI generally implies better access to health services, the large disparity between the two groups can also be explained by flaws in the registration process itself. This could be the case for the State of Rio de Janeiro (RJ) and Federal District (DF), both with high HDI, but with few cases of PC. For these reasons the total number of PC patients registered at the RHC/INCA database is probably underestimated.
There is no specific filter at INCA database to select mCRPC patients or historic data about the PSA values, but the identification of medical procedures the patients had access to and their combinations in order of occurrence (surgery, radiotherapy, chemotherapy, and hormonal therapy) are available. Therefore, to estimate the mCRPC population in Brazil, two filters were selected already described in Methods. PC patients classified as Tx, Nx, M1 (distant metastasis already detected), and undergoing chemotherapy are most likely classified as mCRPC, even though no categorical denomination was determined at RHC/INCA database.
There has been a continuing increase in the number of mCRPC cases registered at the database over time. The percentage of PC patients classified as mCRPC established in this study varied from 1.0% in 2008 up to 2.8% in 2018. This trend is expected due to population aging. However, the effect of the COVID-19 pandemic on the incidence of this disease will be noticed in the coming years, as studies have already indicated a reduction in the life expectancy of the Brazilian population32.
RWD has been used in several countries to estimate the number of mCRPC patients. Thurin et al.18 used the French nationwide healthcare database to estimate the incidence and prevalence of mCRPC in 2014. They found 12,951 mCRPC patients (3.4 %), among with 386,127 PC cases. Wallace et al.33 identified database 343,089 PC patients among the USA population from administrative claims, and an estimated 3,690 mCRPC cases (1.1%). Yu et al.34, utilizing cancer registry data (from 1996 up to 2007), estimated 60,910 PC cases in 2017 and 3% of mCRPC patients in Australia.
Through the analysis of two Brazilian groups stablished herein, the median age of patients in Group 2 was higher than in Group 1. As the Federative Units in Group 2 have the lowest HDI, it is possible that this difference reflects the difficulty of access to the health system, causing a delay in the initial diagnosis.
Although the same percentage of patients with chemotherapy alone as intervention was found in both groups, it seems that more patients were treated with only two therapies in Group 2 (45.28% versus 42.51% in Group 1). This situation is inverted if more therapies are considered. This difference can be related to the higher median age at diagnosis in Group 2 and the resulting lower treatment time.
Furthermore, patients in Group 2 underwent more radiotherapy and surgery than hormone therapy. This may reflect a cultural trait or difficulty in accessing it.
This perception reveals important information about mCRPC patients, their distribution and major therapies used nationwide, and could be utilized to manage resources and public policies. With recent changes in mCRPC patient management (new drugs, imaging techniques, and treatment protocols), estimates of the incidence, prevalence, and other scenarios of this disease can be adopted for health-service planning actions. In this direction, the RWD represents an important tool. This study used a secondary RWD from RHC/INCA that considers standard treatments for the mCRPC patient (symptomatic), as suggested by the Brazilian Guidelines14: hormone therapy (1st and 2nd lines) followed by chemotherapy (docetaxel). Palliative bone pain treatment with the radiopharmaceutical lexidronam (153Sm) is also prescribed when available. Although there are marketing authorizations for other medications, such as abiraterone, enzalutamide and 223Ra, these options are not offered by the Brazilian public health system.
Nevertheless, technological limitations should be pointed out. The patients evaluated in this study probably underwent tomography and/or scintigraphy examinations, whose sensitivity for detecting metastatic disease in early stages is limited and most patients are eventually diagnosed with bone metastasis. The access to more sensitive technologies leads to early detection of metastatic disease (lymph node involvement).
The identification of mCRPC patients is a complex procedure17 which can be indicated as a potential limitation of the present study. Some of the conditions adopted to identify metastatic patients are found in several fields at INCA database, such as the date of the first metastasis, the basis of the diagnosis as metastasis histology, and secondary malignant neoplasm. However, no codification was utilized to define the occurrence of castration resistance. The current estimates were based on the number of treatments the patient was submitted to, however, this is an indirect measure that most likely is underestimated.
The database is validated annually with continuous quality control prior to data publication by INCA. However, there are currently no clear criteria for reviewing the quality of this data, other than periodic audits of a small percentage of the original forms to identify possible discrepancies. It should be noted that reviews of data quality are relevant due to possible errors in filling in similar fields, such as dates of diagnosis, initiation of treatment, first appointment, etc.
RHC/INCA is a general database. Results show that although PC data registration has been continuously growing, essential information related to this cancer is not available, mainly because there are no specific fields to be filled, showing an important weakness of the current database. This work shows that it is critically important to develop specific registering forms for PC and it may also apply to other important types of cancers.
CONCLUSION
This work showed a methodology to estimate the profile of mCRPC patients utilizing publicly available data. The study findings indicated regional disparities in the distribution of registered mCRPC patients across Brazilian territory, revealing two distinct population groups. One of them comprised mostly of Federative Units with higher HDI, while the other consisted of units with lower HDI. The differences found may be attributed to the higher incidence of PC in Federative Units with higher HDI, as supported by literature. However, the under registration of mCRPC cases in Federative Units with lower HDI could also be due to limited access to medical care in those regions.
Additionally, the study revealed regional disparities in the treatment profiles of mCRPC patients in Brazil, which were not in accordance with the current Brazilian guidelines. These differences may be attributed to technological limitations or cultural factors. Nevertheless, this information can be leveraged to strengthen the recently updated guidelines and improve the palliative care provided to mCRPC patients.
Despite the weaknesses in the PC registration process highlighted in this study, the quality of the registration process is expected to improve as the number of registry centers increases. Meanwhile, the study results can be utilized to address regional disparities in patient follow-up by the government. Furthermore, it can help to evaluate the necessity and potential impact of adopting new technologies such as 223Ra or 177Lu-PSMA in a national analysis.
CONTRIBUTIONS
Lorena Pozzo and Mércia Liane de Oliveira contributed to the study design, data collection, analysis, interpretation, and critical review; Lucilena Rebelo Monteiro and Mário Olimpio contributed to data collection, analysis, and interpretation; Marcus Vinícius Sadi and Francesco Giammarile contributed to the critical review. All the authors approved the final version for publication.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interest to declare.
FUNDING SOURCES
None.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-49. doi: https://doi.org/10.3322/caac.21660
2. Instituto Nacional de Câncer. Estimativa 2023: incidência de câncer no Brasil [Internet]. Rio de Janeiro: INCA; 2022 [acesso 2023 abr 12]. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2023.pdf
3. Culp MBB, Soerjomataram I, Efstathiou JA, et al. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77(1):38-52. doi: https://doi.org/10.1016/j.eururo.2019.08.005
4. Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13(8):790-801. doi: https://doi.org/10.1016/S1470-2045(12)70211-5
5. Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243-62. doi: https://doi.org/10.1016/j.eururo.2020.09.042
6. Drazer MW, Stadler WM. The role of testosterone in the treatment of castration resistant prostate cancer. Cancer J. 2016;22(5):330-3. doi: https://doi.org/10.1097/PPO.0000000000000216
7. Nakazawa M, Paller C, Kyprianou N. Mechanisms of therapeutic resistance in prostate cancer. Curr Oncol Rep. 2017;19(2):13. doi: https://doi.org/10.1007/s11912-017-0568-7
8. Gómez-Caamaño A, González-San Segundo C, Henríquez I, et al. Consensus on management of castration-resistant prostate cancer on behalf of the Urological Tumours Working Group (URONCOR) of the Spanish Society of Radiation Oncology. Clin Transl Oncol. 2019;21(4):420-32. doi: https://doi.org/10.1007/s12094-018-1940-2
9. Fox JJ, Gavane SC, Blanc-Autran E, et al. Positron emission tomography/computed tomography-based assessments of androgen receptor expression and glycolytic activity as a prognostic biomarker for metastatic castration-resistant prostate cancer. JAMA Oncol. 2018;4(2):217-24. doi: https://doi.org/10.1001/jamaoncol.2017.3588
10. Gandaglia G, Abdollah F, Schiffmann J, et al. Distribution of metastatic sites in patients with prostate cancer: a population-based analysis. Prostate. 2014;74(2):210-6. doi: https://doi.org/10.1002/pros.22742
11. Nafissi NN, Kosiorek HE, Butterfield RJ, et al. Evolving natural history of metastatic prostate cancer. Cureus. 2020;12(11):e11484. doi: https://doi.org/10.7759/cureus.11484
12. Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091-1103. doi: https://doi.org/10.1056/NEJMoa2107322
13. Handkiewicz-Junak D, Poeppel TD, Bodei L, et al. EANM guidelines for radionuclide therapy of bone metastases with beta-emitting radionuclides. Eur J Nucl Med Mol Imaging. 2018;45(5):846-59. doi: https://doi.org/10.1007/s00259-018-3947-x
14. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Portaria nº 498, de 11 de maio de 2016. Aprova as Diretrizes Diagnósticas e Terapêuticas do Adenocarcinoma de Próstata. Diário Oficial da União, Brasília, DF. 2016 maio12 [acesso 2022 mar 23]; Seção 1:140. Disponível em: https://www.gov.br/conitec/pt-br/midias/relatorios/portaria/2016/ddt_adenocarcinoma_prostata.pdf
15. Beckmann K, Garmo H, Franck Lissbrant I, et al. The value of real-world data in understanding prostate cancer risk and improving clinical care: examples from swedish registries. Cancers (Basel). 2021;13(4):875. doi: https://doi.org/10.3390/cancers13040875
16. Magnani CJ, Bievre N, Baker LC, et al. Real-world evidence to estimate prostate cancer costs for first-line treatment or active surveillance. Eur Urol Open Sci. 2021;23:20-29. doi: https://doi.org/10.1016/j.euros.2020.11.004
17. George DJ, Sartor O, Miller K, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18(4):284-94. doi: https://doi.org/10.1016/j.clgc.2019.12.019
18. Thurin NH, Rouyer M, Gross-Goupil M, et al. Epidemiology of metastatic castration-resistant prostate cancer: a first estimate of incidence and prevalence using the French Nationwide Healthcare Database. Cancer Epidemiol. 2020;69:101833. doi: https://doi.org/10.1016/j.canep.2020.101833
19. Framework for FDA’s real-world evidence program [Internet]. [place unknown]: U.S. Food and Drug Administration; 2018 Dec [cited 2020 Oct 23]. Available from: https://www.fda.gov/media/120060/download
20. Ministério da Saúde (BR). Secretaria de Atenção à Saúde. Portaria nº 741, de 19 de dezembro de 2005. Define as Unidades de Assistência de Alta Complexidade em Oncologia, os Centros de Assistência de Alta Complexidade em Oncologia (CACON) e os Centros de Referência de Alta Complexidade em Oncologia e suas Aptidões e Qualidades. Diário Oficial da União, Brasília, DF. 2005 dez 23 [acesso 2022 out 21]; Seção 1:113. Disponível em: http://bvsms.saude.gov.br/bvs/saudelegis/sas/2005/prt0741_19_12_2005.html
21. Integrador RHC: Registros Hospitalares de Câncer [Internet]. Rio de Janeiro: INCA. [2012] – [acesso 2021 maio 15]. Disponível em: https://irhc.inca.gov.br
22. CNES: Cadastro Nacional de Estabelecimentos de Saúde [Internet]. Brasília (DF): DATASUS. [2000] – [acesso 2022 out 21]. Disponível em: http://cnes.datasus.gov.br/
23. Conselho Nacional de Saúde (BR). Resolução nº 510, de 7 de abril de 2016. Dispõe sobre as normas aplicáveis a pesquisas em Ciências Humanas e Sociais cujos procedimentos metodológicos envolvam a utilização de dados diretamente obtidos com os participantes ou de informações identificáveis ou que possam acarretar riscos maiores do que os existentes na vida cotidiana, na forma definida nesta Resolução [Internet]. Diário Oficial da União, Brasília, DF. 2016 maio 24 [acesso 2021 maio 15]; Seção 1:44. Disponível em: http://bvsms.saude.gov.br/bvs/saudelegis/cns/2016/res0510_07_04_2016.html
24. Instituto Brasileiro de Geografia e Estatística [Internet]. Rio de janeiro: IBGE; c2023. Censo 2010; [acesso 2022 mar 23]. Disponível em: https://censo2010.ibge.gov.br/sinopse/index.php?dados=8
25. Silberschatz A, Korth HF, Sudarshan S. Database system concepts. 6th ed. New York, NY: McGraw Hill Education; 2010.
26. Teoh JYC, Hirai HW, Ho JMW, et al. Global incidence of prostate cancer in developing and developed countries with changing age structures. PLoS One. 2019;14(10):e0221775. doi: https://doi.org/10.1371/journal.pone.0221775
27. Mori RR, Faria EF, Mauad EC, et al. Prostate cancer screening among elderly men in Brazil: should we diagnose or not? Int Braz J Urol. 2020;46(1):34-41. doi: https://doi.org/10.1590/S1677-5538.IBJU.2019.0022
28. Braga SFM, Souza MC, Cherchiglia ML. Time trends for prostate cancer mortality in Brazil and its geographic regions: an age-period-cohort analysis. Cancer Epidemiol. 2017;50(Pt A):53-59. doi: https://doi.org/10.1016/j.canep.2017.07.016
29. Romero FR, Romero AW, Almeida RMS, et al. The prevalence of prostate cancer in Brazil is higher in Black men than in White men: systematic review and meta-analysis. Int Braz J Urol. 2012;38(4):440-7. doi: https://doi.org/10.1590/s1677-55382012000400002
30. Silva GA, Jardim BC, Ferreira VM, et al. Cancer mortality in the Capitals and in the interior of Brazil: a four-decade analysis. Rev Saúde Pública. 2020;54:126. doi: https://doi.org/10.11606/s1518-8787.2020054002255
31. Instituto Brasileiro de Geografia e Estatística. Tábua completa de mortalidade para o Brasil – 2018: breve análise da evolução da mortalidade no Brasil [Internet]. Rio de Janeiro: IBGE; 2019 [2022 mar 3]. Disponível em: https://biblioteca.ibge.gov.br/visualizacao/periodicos/3097/tcmb_2018.pdf
32. Castro MC, Gurzenda S, Turra CM, et al. Reduction in life expectancy in Brazil after COVID-19. Nat Med. 2021;27(9):1629-35. doi: https://doi.org/10.1038/s41591-021-01437-z
33. Wallace K, Landsteiner A, Bunner S, et al. Epidemiology and mortality of metastatic castration-resistant prostate cancer (mCRPC) in a managed care population in the United States. J Clin Oncol. 2020;38(15 Suppl):e13592. doi: https://doi.org/10.1200/JCO.2020.38.15_suppl.e13592
34. Yu XQ, Luo Q, Smith DP, et al. Phase of care prevalence for prostate cancer in New South Wales, Australia: a population-based modelling study. PLoS One. 2017;12(2):e0171013. doi: https://doi.org/10.1371/journal.pone.0171013
Recebido em 6/2/2023
Aprovado em 2/5/2023
Associate-Editor: Jeane Tomazelli. Orcid iD: https://orcid.org/0000-0002-2472-3444
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde