ORIGINAL ARTICLE
Influence of Age on Health-Related Quality of Life of Women Diagnosed with Breast Cancer
Influência da Idade na Qualidade de Vida Relacionada à Saúde de Mulheres Diagnosticadas com Câncer de Mama
Influencia de la Edad en la Calidad de Vida Relacionada con la Salud de Mujeres Diagnosticadas con Cáncer de Mama
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n2.3826
Larissa Nascimento dos Santos1; Suzana Sales de Aguiar2; Graziele Marques Rodrigues3; Luiz Claudio Santos Thuler4; Anke Bergmann5
1-5Instituto Nacional de Câncer (INCA), Coordenação de Pesquisa Clínica, Divisão de Pesquisa Clínica e Desenvolvimento Tecnológico. Rio de Janeiro (RJ), Brazil. E-mails: larissadossantosn@gmail.com; saguiar@inca.gov.br; grazi_rodrigues06@hotmail.com; lthuler@inca.gov.br; abergmann@inca.gov.br. Orcid iD: https://orcid.org/0000-0003-2114-8840; Orcid iD: https://orcid.org/0000-0003-1963-1294; Orcid iD: https://orcid.org/0000-0002-4299-2349; Orcid iD: https://orcid.org/0000-0003-2550-6537; Orcid iD: https://orcid.org/0000-0002-1972-8777
Corresponding author: Suzana Sales de Aguiar. Rua André Cavalcanti, 37 – Sala 8, Anexo – Centro. Rio de Janeiro (RJ), Brazil. CEP 20231-050. E-mail: saguiar@inca.gov.br
ABSTRACT
Introduction: Women with breast cancer may have differences in health-related quality of life (HRQoL) at diagnosis by age. Objective: To analyze the influence of age on the HRQoL of women diagnosed with breast cancer. Method: Cross-sectional study was carried out with women diagnosed with breast cancer. HRQoL assessment was performed before starting cancer treatment, using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and its specific breast cancer module (BR-23). Association between age group and HRQoL was determined through multiple linear regression. Results: 961 women were included in the study, with a mean age of 54 (SD±11.7). Women aged ≥50 years displayed better emotional functioning (+7.6 points; p<0.001), and less fatigue (-4.4 points; p=0.014), pain (-4.7 points; p=0.033), nausea and vomiting (-2.3 points; p=0.030) and financial difficulties (-10.3 points; p<0.001) compared to younger women. Concerning the BR-23 module, these women displayed better body image scores (+3.6 points; p=0.029) and future perspective (+12.4 points; p<0.001), and worse sexual functioning (-19.9 points; p<0.001) and sexual enjoyment (-8.9 points; p=0.001), and on the symptom scale, less breast symptoms (-11.6 points; p<0.001) and arm symptoms (-3.5 points; p=0.047). Conclusion: Patients aged ≥50 years exhibited better HRQoL in all QLQ C-30 and BR-23 functioning scales and symptom scales, except for sexual functioning and sexual enjoyment.
Key words: breast neoplasms; quality of life; surveys and questionnaires; age factors.
RESUMO
Introdução: Mulheres com câncer de mama podem apresentar diferenças na qualidade de vida relacionada à saúde (QVRS) ao diagnóstico de acordo com a idade. Objetivo: Analisar a influência da idade na QVRS de mulheres com diagnóstico de câncer de mama. Método: Estudo transversal com mulheres diagnosticadas com câncer de mama. A avaliação da QVRS foi realizada antes do início do tratamento oncológico por meio do questionário European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) e seu módulo específico para o câncer de mama (BR-23). A associação entre faixa etária e QVRS foi determinada por meio da regressão linear múltipla. Resultados: Foram incluídas 961 mulheres com média de idade de 54 anos (DP±11,7). Mulheres com idade ≥50 anos apresentaram melhor função emocional (+7,6 pontos; p<0,001), menos fadiga (-4,4 pontos; p=0,014), dor (-4,7 pontos; p=0,033), náuseas e vômitos (-2,3 pontos; p=0,030) e dificuldade financeira (-10,3 pontos; p<0,001) comparadas às mulheres jovens. Em relação ao BR-23, essas mulheres apresentaram melhor escores de imagem corporal (+3,6 pontos; p=0,029) e de perspectiva futura (+12,4 pontos; p<0,001), e piores função sexual (-19,9 pontos; p<0,001) e satisfação sexual (-8,9 pontos; p=0,001); e, na escala de sintomas, menos sintomas na mama (-11,6 pontos; p<0,001) e sintomas no braço (-3,5 pontos; p=0,047). Conclusão: As pacientes com idade ≥50 anos apresentaram melhor QVRS em todos os domínios das escalas de função e escalas de sintomas do QLQ C-30 e BR-23, exceto no que diz respeito à função sexual e à satisfação sexual.
Palavras-chave: neoplasias da mama; qualidade de vida; inquéritos e questionários; fatores etários.
RESUMEN
Introducción: Las mujeres con cáncer de mama pueden tener diferencias en la calidad de vida relacionada con la salud (CVRS) al momento del diagnóstico según la edad. Objetivo: Analizar la influencia de la edad en la CVRS de mujeres con diagnóstico de cáncer de mama. Método: Estudio transversal con mujeres diagnosticadas con cáncer de mama. La evaluación de la CVRS se realizó antes de iniciar el tratamiento oncológico mediante el Cuestionario European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) y su módulo específico para el cáncer de mama (BR-23). La asociación entre el grupo de edad y la CVRS se determinó mediante regresión lineal múltiple. Resultados: Se incluyeron 961 mujeres con una edad media de 54 años (DE±11,7). Las mujeres ≥50 años tuvieron mejor funcionamiento emocional (+7,6 puntos; p<0,001), y menos fatiga (-4,4 puntos; p=0,014), dolor (-4,7 puntos; p=0,033), náuseas y vómitos (-2,3 puntos; p=0,030) y dificultades financieras (-10,31 puntos; p<0,001) en comparación con las mujeres jóvenes. Con respecto al BR-23, estas mujeres presentaron mejores puntajes de imagen corporal (+3,6 puntos; p=0,029) y perspectiva de futuro (+12,4 puntos; p<0,001) y peor función sexual (-19,9 puntos; p<0,001) y satisfacción sexual (-8,9 puntos; p=0,001), y en la escala de síntomas, menos síntomas mamarios (-11,6 puntos; p<0,001) y brazos (-3,5 puntos; p=0,047). Conclusión: Las pacientes con edad ≥50 años tuvieron mejor CVRS en todos los dominios de las escalas de función y síntomas del QLQ C-30 y BR-23, excepto función sexual y satisfacción sexual.
Palabras clave: neoplasias de la mama; calidad de vida; encuestas y cuestionarios; factores de edad.
INTRODUCTION
Breast cancer results in the highest incidence and mortality rates in the female population worldwide among the different types of cancer1. The diagnosis of a disease comprising a high risk of death can lead to psychological symptoms, anxiety and depression, negatively impacting the health-related quality of life (HRQoL) of this population2-5.
The HRQoL has been routinely applied as a health indicator due to its association with mortality, treatment effectiveness and survival of women with breast cancer6-8.
Breast cancer and age exhibit a well-established association in scientific studies. Aging is an inherent risk factor for the increased incidence and mortality of this disease9, and some authors also consider this variable as associated with worse HRQoL10-11. However, conflicting results have been reported by other authors, who observed that younger women diagnosed with breast cancer exhibit worse HRQoL12. In addition, younger patients more often report symptoms such as fear, anxiety, depression and problems with body self-image, which can interfere with their HRQoL13,14. A cross-sectional study conducted on breast cancer survivors indicates that cancer stage or treatments do not impact HRQoL in young women but instead, affect issues related to fertility, sexuality and professional reintegration15.
Faced with these controversial results, this study aims to analyze the influence of age on the HRQoL of women diagnosed with breast cancer. Based on the age of these women and their quality of life, it is possible to achieve better management of specific needs of each age group.
METHOD
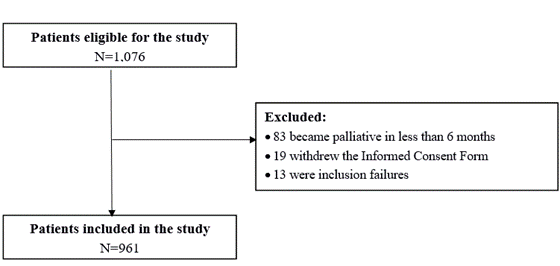
A cross-sectional study was carried out with women diagnosed with breast cancer (ICD-10 C50) aged 18 or over undergoing curative-intent treatment (stages I, II and III) and enrolled at the Hospital do Câncer III (HCIII)/Instituto Nacional de Câncer (INCA), Rio de Janeiro, Brazil, from April 4, 2016 to April 30, 2019. Women with a diagnosis of distant metastasis up to six months after recruitment, those that withdraw their Informed Consent Form and study inclusion failures were excluded from the evaluation (Figure 1).
|
|
|
Figure 1. Study population flowchart |
Patients were enrolled after admitted at the hospital, prior to beginning the cancer treatment, in the first appointment with the oncologist or in the preoperative period for breast cancer surgery. Eligible women were invited to participate in the study and were submitted to an interview, physical examination and application of HRQoL questionnaires after signing the consent form.
The main exposure in this study was age at recruitment, assessed in a dichotomous manner with a cutoff point set at 50 years of age, as this is the beginning of the age group at the highest risk for the disease in the country, reported as women aged between 50 and 69 years old.
The outcome (HRQoL) was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and its specific breast cancer module (BR-23), both translated and validated for the Brazilian population16,17.
The EORTC QLQ-C30 questionnaire comprises 30 questions that aim to assess HRQoL in the 7 days prior to its application. It is categorized into functional scale (physical functioning, role functioning, cognitive functioning, emotional functioning and social functioning) and symptoms/items scale (fatigue, pain, dyspnea, insomnia, appetite loss, nausea and vomiting, constipation, diarrhea and financial difficulties), with responses ranging from 1 to 4 (1 – not at all, 2 – a little, 3 – quite a bit, 4 – very much). It also presents a general health and global quality of life scale, with response options ranging from 1 to 7, with 1 being very poor and 7, excellent.
The EORTC QLQ-BR23 questionnaire comprises 23 questions and is also intended to assess HRQoL in the 7 days prior to its application. It is categorized into two dimensions, comprising a functional scale (body image, sexual functioning, sexual enjoyment and future perspective) and symptoms/items (systemic therapy side effects, breast symptoms, arm symptoms and upset by hair loss) scale, with response options ranging from 1 to 4 (1 – not at all, 2 – a little, 3 – quite a bit, 4 – very much).
At the study enrollment, sociodemographic and lifestyle covariates (race/skin color, marital status, education, alcohol use in the last 30 days and current tobacco use), clinical covariates (hypertension, status menopausal, body mass index) and tumor covariates (clinical stage, histological type) were obtained. The variables were collected through interviews and physical assessment, except for arterial hypertension, clinical stage and histological type, which were collected through a direct search of physical and electronic medical records.
A descriptive analysis of the population was performed through central tendency and dispersion measures for continuous variables and frequency distribution for categorical variables. Student's t test was used to evaluate comparisons of the means of each domain by age group for HRQoL.
Associations between age group and HRQoL were determined by applying a simple linear regression. To control the confounding variables, covariates with p<0.20 in the simple linear regression were selected for the multiple model, applying the stepwise forward method. A p-value of <0.05 was considered statistically significant and set for all the analyses performed with SPSS 23.0 program. In compliance with Resolution 466/201218 of the National Health Council, the Institutional Review Board of INCA approved the study, CAAE (Submission for Ethical Review) 51100615.7.0000.5274.
RESULTS
A total of 961 women were included in the study, with a mean age of 54 (SD±11.7) and median of 55 years old (range 23 to 86). Most women were Brown (40.8%), with over 8 years of education (69.0%). The predominant histological type was infiltrating ductal carcinoma (83.5%) and most patients (54.4%) were diagnosed with advanced clinical stage breast cancer (≥II B) (Table 1).
Table 1. Sociodemographic and clinical characteristics of the study population (N=961)
|
Total 961 (100%) |
<50 years old 364 (37.9%) |
≥50 years old 597 (62.1%) |
||||
|
N |
% |
N |
(%) |
N |
(%) |
|
|
Race/skin color |
|
|
|
|
|
|
|
White |
337 |
35.1 |
117 |
32.1 |
220 |
36.9 |
|
Black |
204 |
21.2 |
75 |
20.6 |
129 |
21.6 |
|
Brown |
392 |
40.8 |
158 |
43.4 |
234 |
39.2 |
|
Yellow/Indigenous |
28 |
2.9 |
14 |
3.9 |
14 |
2.3 |
|
Marital status |
|
|
|
|
|
|
|
With spouse |
485 |
50.4 |
205 |
56.3 |
280 |
43.9 |
|
Without spouse |
476 |
49.6 |
159 |
43.7 |
317 |
53.1 |
|
Years of study |
|
|
|
|
|
|
|
<8 |
298 |
31.0 |
69 |
19.0 |
229 |
38.4 |
|
≥8 |
663 |
69.0 |
295 |
81.0 |
368 |
61.6 |
|
Alcohol use in the last 30 days |
|
|
|
|
|
|
|
Yes |
249 |
25.9 |
104 |
28.6 |
145 |
24.3 |
|
No |
709 |
73.8 |
258 |
70.9 |
451 |
75.5 |
|
No information |
3 |
0.3 |
2 |
0,5 |
1 |
0.2 |
|
Current tobacco use |
|
|
|
|
|
|
|
Does not smoke |
651 |
67.7 |
289 |
79.4 |
362 |
60.6 |
|
Smoker |
85 |
8.8 |
30 |
8.2 |
55 |
9.2 |
|
Ex-smoker |
221 |
23.0 |
43 |
11.8 |
178 |
29.8 |
|
No information |
4 |
0.5 |
2 |
0.5 |
2 |
0.4 |
|
Arterial hypertension |
|
|
|
|
|
|
|
Yes |
433 |
45.1 |
80 |
22.0 |
353 |
59.1 |
|
No |
526 |
54.7 |
284 |
78.0 |
242 |
40.5 |
|
No information |
2 |
0.2 |
0 |
0.0 |
2 |
0.4 |
|
Menopausal status |
|
|
|
|
|
|
|
Post-menopause |
600 |
62.4 |
54 |
14.8 |
546 |
91.5 |
|
Premenopause |
334 |
34.8 |
299 |
82.2 |
35 |
5.9 |
|
No information |
27 |
2.8 |
11 |
3.0 |
16 |
2.6 |
|
Body mass index |
|
|
|
|
|
|
|
Low weight |
11 |
1.1 |
5 |
1.4 |
6 |
1.0 |
|
Eutrophic |
222 |
23.1 |
90 |
24.7 |
132 |
22.1 |
|
Overweight |
358 |
37.3 |
131 |
36.0 |
227 |
38.0 |
|
Obesity |
344 |
35.8 |
131 |
36.0 |
213 |
35.7 |
|
No information |
26 |
2.7 |
7 |
1.9 |
19 |
3.2 |
|
Clinical staging |
|
|
|
|
|
|
|
<IIB |
418 |
43.5 |
110 |
30.2 |
308 |
51.6 |
|
≥IIB |
523 |
54.4 |
248 |
68.1 |
275 |
46.1 |
|
No information |
20 |
2.1 |
6 |
1.6 |
14 |
2.3 |
|
Histological tumor type |
|
|
|
|
|
|
|
IDC |
802 |
83.5 |
317 |
87.1 |
485 |
81.2 |
|
Others |
148 |
15.4 |
45 |
12.4 |
103 |
17.3 |
|
No information |
11 |
1.1 |
2 |
0.5 |
9 |
1.5 |
|
Captions: SD = Standard deviation; IDC = Infiltrating ductal carcinoma. |
||||||
Based in the EORTC QLQ C-30 questionnaire, patients aged ≥50 exhibited better social (p=0.015) and emotional (p<0.001) functioning and less frequent fatigue (p=0.002), pain (p=0.001), nausea and vomiting (p=0.039) and financial difficulties (p=0.001) compared to women under 50 years old. The specific EORTC QLQ BR-23 breast cancer module revealed that patients aged ≥50 reported better body image (p=0.015) and future perspectives (p<0.001), although quality of life was negatively affected for sexual functioning (p<0.001) and sexual enjoyment (p<0.001). Patients aged ≥50 presented fewer breast (p<0.001) and arm (p=0.040) symptoms (Table 2).
Table 2. Descriptive analysis of HRQoL by age group and mean difference between the two groups (N=961)
|
Means (±SD) N=961 |
Means (±SD) N=364 |
Means (±SD) N=597 |
Mean difference between groups (CI 95%) |
p-value |
|
|
EORTC QLQ C-30 |
Total |
<50 years old |
≥50 years old |
|
|
|
Functional scales |
|
|
|
|
|
|
Global health status |
69.5 (23.3) |
67.7 (23.0) |
70.5 (23.5) |
2.8 (-0.3 to 5.8) |
0.075 |
|
Physical functioning |
83.0 (19.8) |
82.6 (20.3) |
83.3 (19.5) |
0.7 (-1.8 to 3.3) |
0.596 |
|
Role functioning |
78.5 (30.5) |
76.4 (31.4) |
79.7 (29.9) |
3.3 (-0.7 to 7.3) |
0.105 |
|
Cognitive functioning |
74.3 (28.7) |
73.3 (31.1) |
75.0 (27.2) |
1.6 (-2.1 to 5.4) |
0.393 |
|
Emotional functioning |
56.2 (31.4) |
50.6 (31.8) |
59.6 (30.7) |
9.0 (4.9 to 13.1) |
<0.001 |
|
Social functioning |
81.1 (29.8) |
78.1 (30.3) |
82.9 (29.8) |
4.8 (0.9 to 8.7) |
0.015 |
|
Symptom scales/items |
|
|
|
|
|
|
Fatigue |
22.7 (26.2) |
26.1 (26.9) |
20.6 (25.5) |
-5.5 (-8.9 to -2.08) |
0.002 |
|
Pain |
31.4 (32.9) |
35.8 (32.9) |
28.7 (32.7) |
-7.1 (-11.4 to -2.8) |
0.001 |
|
Dyspnea |
11.4 (24.7) |
11.9 (24.1) |
11.0 (25.1) |
-0.9(-4.2 to 2.3) |
0.577 |
|
Insomnia |
37.6 (41.8) |
37.2 (41.8) |
37.8 (41.9) |
0.6 (-4.9 to 6.1) |
0.834 |
|
Appetite loss |
14.2 (29.0) |
14.7 (29.0) |
13.9 (29.1) |
-0.8 (-4.6 to 3.0) |
0.671 |
|
Nausea and vomiting |
7.4 (15.9) |
8.7 (17.8) |
6.5 (14.6) |
-2.2 (-4.3 to -0.1) |
0.039 |
|
Constipation |
19.0 (32.9) |
20.2 (33.5) |
18.2 (32.5) |
-2.0 (-6.3 to 2.3) |
0.355 |
|
Diarrhea |
6.6 (19.2) |
5.9 (17.6) |
7.0 (20.2) |
1.1 (-1.4 to 3.6) |
0.400 |
|
Financial difficulties |
28.7 (39.9) |
34.2 (41.9) |
25.4 (38.6) |
-8.7 (-13.9 to -3.5) |
0.001 |
|
EORTC BR-23 |
|
|
|
|
|
|
Functional scales |
|
|
|
|
|
|
Body image |
83.2 (24.8) |
80.7 (26.1) |
84.7 (24.0) |
4.0 (0.8 to 7.3) |
0.015 |
|
Sexual functioning |
33.1 (31.5) |
48.2 (31.7) |
23.9 (27.6) |
-24.4 (-28.2 to -20.5) |
<0.001 |
|
Sexual enjoyment |
71.8 (29.1) |
76.3 (27.4) |
66.3 (30.2) |
-9.9 (-15.2 to -4.7) |
<0.001 |
|
Future perspective |
35.6 (39.1) |
26.8 (35.3) |
41.0 (40.3) |
14.2 (9.2 to 19.3) |
<0.001 |
|
Symptoms scales/items |
|
|
|
|
|
|
Systemic therapy side effects |
19.2 (17.5) |
19.8 (17.5) |
18.9 (17.5) |
-0.9 (-3.2 to 1.4) |
0.453 |
|
Upset by hair loss |
34.6 (42.1) |
36.8 (41.1) |
33.1 (42.8) |
-3.8 (-14.8 to 7.3) |
0.504 |
|
Breast symptoms |
28.9 (29.8) |
39.0 (32.1) |
22.8 (26.5) |
-16.2 (-20.0 to -12.5) |
<0.001 |
|
Arm symptoms |
18.2 (24.3) |
20.3 (26.0) |
17.0 (23.0) |
-3.3 (-6.5 to -0.1) |
0.040 |
|
Captions: CI = confidence interval; SD = standard deviation; EORTC QLQ C-30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EORTC BR-23 = European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life Questionnaire (QLQ-BR23). Note: p-values<0.05 are highlighted in bold; Functioning scale: the higher the score, better is the HRQoL. Symptom scale: the higher the score, worse is the HRQoL. |
|||||
After adjusting for potential confounding variables, the HRQoL assessed by the QLQ C-30 indicated that women aged ≥50 exhibited better emotional functioning (7.6 points; p<0.001), and less fatigue (-4,4 points; p=0.014), pain (-4.7 points; p=0.033), nausea and vomiting (-2.3 points; p=0.030) and financial difficulties (-10.31 points; p<0.001) compared to younger women. When evaluating the HRQoL applying the BR-23 module, women aged ≥50 exhibited better HRQoL in relation to body image (3.6 points; p=0.029) and future perspective scores (12.4 points; p<0.001), but worse sexual functioning (-19.9 points; p<0.001) and sexual enjoyment (-8.9 points; p=0.001). The symptoms scale revealed fewer breast symptoms (-11.6 points; p<0.001) and arm symptoms (-3.5 points; p=0.047) for women younger than 50 years of age (Table 3).
Table 3. Association between age ≥50 years and HRQoL domains (N=961)
|
Beta |
CI 95% |
p-value |
|
|
EORTC QLQ C-30 |
|
|
|
|
Functional scales |
|
|
|
|
Emotional functioninga |
7.6 |
(3.4 to 11.9) |
<0.001 |
|
Symptoms scales / items |
|
|
|
|
Fatiguea |
-4.4 |
(-8.0 to -0.9) |
0.014 |
|
Painb |
-4.7 |
(-9.0 to -0.4) |
0.033 |
|
Nausea and vomitingc |
-2.3 |
(- 4.4 to -0.2) |
0.030 |
|
Financial difficultiesd |
-10.3 |
(-15.6 to -5.0) |
<0.001 |
|
EORTC BR-23 |
|
|
|
|
Functional scales |
|
|
|
|
Body imagee |
3.6 |
(0.3 to 6.9) |
0.029 |
|
Sexual functioningf |
-19.9 |
(-24.0 to -15.9) |
<0.001 |
|
Sexual enjoymentg |
-8.9 |
(-14.2 to -3.5) |
0.001 |
|
Future perspectiveh |
12.4 |
(7.2 to 17.6) |
<0.001 |
|
Symptoms scales / items |
|
|
|
|
Breast symptomsi |
-11.6 |
(-15.2 to -8.0) |
<0.001 |
|
Arm symptomsj |
-3.5 |
(-6.9 to -0.05) |
0.047 |
|
Captions: CI = confidence interval; EORTC QLQ C-30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; EORTC BR-23 = European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life Questionnaire (QLQ-BR23). (a) adjusted by clinical staging and BMI. (b) adjusted by clinical staging, BMI and race. (c) adjusted by BMI. (d) adjusted by marital status. (e) adjusted by BMI. (f) adjusted by race, marital status, education, alcohol use and hypertension. (g) adjusted by education. (h) adjusted by alcohol use and clinical staging. (i) adjusted by clinical staging. (j) adjusted by hypertension and clinical staging. Note: p-values<0.05 are highlighted in bold. Functioning scale; the higher the score, better is the HRQoL. Symptom scale: the higher the score, worse is the HRQoL. |
|||
DISCUSSION
In the present study, most women were diagnosed at advanced breast cancer stages (≥IIB), with a mean age of 54. Before beginning cancer treatment, patients aged ≥50 years exhibited better emotional function, body image and future perspectives and worse sexual function and sexual satisfaction compared to younger patients. Women aged ≥50 years exhibited less fatigue, pain, nausea and vomiting, financial difficulties, breast and arm symptoms.
A study carried out with Vietnamese women, with an age cutoff of 45 years concluded that younger women exhibited better HRQoL19. Another study with 1,498 breast cancer patients analyzed the HRQoL as a function of different stages and age groups and demonstrated that women in the initial stage aged 50 or less exhibited worse overall quality of life scores, as well as in other five domains, when compared to other age groups (p<0.05)20. The Carolina Breast Cancer Study (a study about the causes, treatments and personal experience of North Carolina women diagnosed with breast cancer) with 2,142 women with breast cancer analyzed the profiles of quality-of-life and 5 and 25 months after the diagnosis. Younger women at the diagnosis (OR 0.95; 95% CI 0.93-0.96)21 exhibited the worst quality of life in all domains.
A systematic review22 revealed that younger women treated for breast cancer displayed psychological impacts, weight gain and physical inactivity during treatment, in addition to anxiety and depression, contributing to compromised quality of life. Leinert et al.23 found a higher frequency of fatigue in patients over 60 years of age and a higher prevalence of symptoms such as nausea and vomiting in younger women. It is possible that younger women, while faced with a diagnosis that can bring disabilities or uncertainties about the maintenance of their role in society, feel more threatened compared to older women, who display greater stability for being older, as observed in the present study, where younger women exhibited worse HRQoL for most domains, except sexual function and sexual satisfaction.
A French study also stratified by age group, found that older ages are more associated with economic deprivation or unsatisfactory financial situation, as well as less social support for this population24. In Brazil, a recent study revealed that most younger25 women diagnosed with breast cancer who were employed and/or actively working and more financially independent, may become more emotionally vulnerable when temporary discontinuation of their labor activities may occur due to cancer treatment compared to older women who are less economically active or who do not work full time.
A Saudi Arabian study applied the EORTC instrument to evaluate 284 women already treated for breast cancer, with an average age over 50, and revealed worse HRQoL in relation to the symptom scale26 of sexual function, as the results reported herein have also shown. In addition to hormonal changes resulting from age, a breast cancer diagnosis reduces the rate of female sexual function27. In fact, younger women are often more sexually active than older women, and hormonal factors hold a direct influence on sexual behavior. Thus, younger patients tend to display better sexual function and sexual satisfaction.
Although some differences are noted in the population, age stratification and types of questionnaires used in some studies, most of them report that younger women with breast cancer exhibit a worse quality of life in several domains.
The results presented herein must be considered under the perspective of the study’s strengths and weaknesses which was carried out in a public breast cancer treatment reference hospital in the state of Rio de Janeiro, Brazil, including a high number of patients treated free of charge by the National Health System (SUS). To carry out the interviews, the research team was periodically trained, and questionnaires translated and validated for the Brazilian population were used. Among the limitations of the study is the non-inclusion of important variables as the relationship between age and quality of life, other comorbidities and physical activity. However, the extrapolation of the results to other populations should be done cautiously, considering that the reality of patients treated at a reference center may not reflect the HRQoL profile of women treated at other centers.
This study calls for better evaluation of the sexuality of women diagnosed with breast cancer aged ≥50, with specific quality of life questionnaires, and an investigation on their function and sexual satisfaction for possible treatment and potential improvement in these domains because of the worst scores found in comparison with younger women.
CONCLUSION
Women diagnosed with breast cancer aged ≥50, despite exhibiting worse sexual functioning and sexual enjoyment, presented better emotional functioning, body image and future perspectives, in addition to less pain, fatigue, nausea and vomiting, financial difficulties and breast and arm symptoms.
The differences observed by age group (<50 and >50) indicate the domains in each population requiring interventions to improve the HRQoL of these women, preventing negative physical and mental health effects during all cancer care stages.
CONTRIBUTIONS
Larissa Nascimento dos Santos contributed to the investigation, data curation, formal analysis and wording of the original draft. Suzana Sales de Aguiar contributed to the investigation, project administration and data curation. Graziele Marques Rodrigues contributed to the investigation, data curation, formal analysis. Luiz Claudio Santos Thuler and Anke Bergmann contributed to the study design, methodology, validation, formal analysis, wording, review, editing and supervision. All the authors approved the final version for publication.
DECLARATION OF CONFLICT OF INTERESTS
The author Anke Bergmann declares potential conflict of interests due to its position as Scientific-Editor of INCA’s Revista Brasileira de Cancerologia. The other authors have no conflict of interests to declare
FUNDING SOURCES
Instituto Nacional de Câncer (INCA).
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-49. doi: https://doi.org/10.3322/caac.21660
2. Tsaras K, Papathanasiou IV, Mitsi D, et al. Assessment of depression and anxiety in breast cancer patients: prevalence and associated factors. Asian Pac J Cancer Prev. 2018;19(6):1661-69. doi: https://doi.org/10.22034/APJCP.2018.19.6.1661
3. Brunault P, Champagne AL, Huguet G, et al. Major depressive disorder, personality disorders, and coping strategies are independent risk factors for lower quality of life in non-metastatic breast cancer patients. Psychooncology. 2016;25(5):513-20. doi: https://doi.org/10.1002/pon.3947
4. Gold M, Dunn LB, Phoenix B, et al. Co-occurrence of anxiety and depressive symptoms following breast cancer surgery and its impact on quality of life. Eur J Oncol Nurs. 2016;20:97-105. doi: https://doi.org/10.1016/j.ejon.2015.06.003
5. Cho YU, Lee BG, Kim SH. Coping style at diagnosis and its association with subsequent health-related quality of life in women with breast cancer: a 3-year follow-up study. Eur J Oncol Nurs. 2020;45:101726. doi: https://doi.org/10.1016/j.ejon.2020.101726
6. De Aguiar SS, Bergmann A, Mattos IE. Quality of life as a predictor of overall survival after breast cancer treatment. Qual Life Res. 2014;23(2):627-37. doi: https://doi.org/10.1007/s11136-013-0476-8
7. Karlsen RV, Frederiksen K, Larsen MB, et al. The impact of a breast cancer diagnosis on health-related quality of life. A prospective comparison among middle-aged to elderly women with and without breast cancer. Acta Oncol. 2016;55(6):720-7. doi: https://doi.org/10.3109/0284186X.2015.1127415
8. Mierzynska J, Taye M, Pe M, et al. Reference values for the EORTC QLQ-C30 in early and metastatic breast cancer. Eur J Cancer. 2020;125:69-82. doi: https://doi.org/10.1016/J.Ejca.2019.10.031
9. Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg Oncol Clin N Am. 2018;27(4):675-84. doi: https://doi.org/10.1016/j.soc.2018.05.008
10. Sinha R, Coyle C, Ring A. Breast cancer in older patients: national cancer registry data. Int J Clin Pract. 2013;67(7):698-700. doi: https://doi.org/10.1111/ijcp.12117
11. Park JH, Jung YS, Kim JY, et al. Trajectories of health-related quality of life in breast cancer patients. Support Care Cancer. 2020;28(7):3381-9. doi: https://doi.org/10.1007/s00520-019-05184-3
12. Tejeda S, Stolley MR, Vijayasiri G, et al. Negative psychological consequences of breast cancer among recently diagnosed ethnically diverse women. Psychooncology. 2017;26(12):2245-52. doi: https://doi.org/10.1002/pon.4456
13. Champion VL, Wagner LI, Monahan PO, et al. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer. 2014;120(15):2237-46. doi: https://doi.org/10.1002/cncr.28737
14. Wöckel A, Schwentner L, Krockenberger M, et al. Predictors of the course of quality of life during therapy in women with primary breast cancer. Qual Life Res. 2017;26(8):2201-8. doi: https://doi.org/10.1007/s11136-017-1570-0
15. Assogba ELF, Kamga AM, Costaz H, et al. What are young women living conditions after breast cancer? Health-related quality of life, sexual and fertility issues, professional reinsertion. Cancers (Basel). 2020;12(6):1564. doi: https://doi.org/10.3390/cancers12061564
16. Michels FAS, Latorre MR, Maciel MS. Validity, reliability and understanding of the EORTC-C30 and EORTC-BR23, quality of life questionnaires specific for breast cancer. Rev Bras Epidemiol. 2013;16(2):352-63. doi: https://doi.org/10.1590/S1415-790X2013000200011
17. Fayers P, Bottomley A; EORTC Quality of Life Group, et al. Quality of life research within the EORTC-the EORTC QLQ-C30. Eur J Cancer. 2002;38(Suppl 4):S125-33. doi: https://doi.org/10.1016/s0959-8049(01)00448-8
18. Conselho Nacional de Saúde (BR). Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília, DF. 2013 jun 13; Seção 1:59.
19. Tran TH, Trinh NL, Hoang Y, et al. Health-related quality of life among vietnamese breast cancer women. Cancer Control. 2019;26(1):1073274819862787. doi: https://doi.org/10.1177/1073274819862787
20. Hamer J, McDonald R, Zhang L, et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support Care Cancer. 2017;25(2):409-19. doi: https://doi.org/10.1007/s00520-016-3417-6
21. Pinheiro LC, Tan X, Olshan AF, et al. Examining health-related quality of life patterns in women with breast cancer. Qual Life Res. 2017;26(7):1733-43. doi: https://doi.org/10.1007/s11136-017-1533-5
22. Howard-Anderson J, Ganz PA, Bower JE, et al. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(5):386-405. doi: https://doi.org/10.1093/jnci/djr541
23. Leinert E, Singer S, Janni W, et al. The impact of age on quality of life in breast cancer patients receiving adjuvant chemotherapy: a comparative analysis from the prospective multicenter randomized ADEBAR trial. Clin Breast Cancer. 2017;17(2):100-6. doi: https://doi.org/10.1016/j.clbc.2016.10.008
24. Dialla PO, Chu WO, Roignot P, et al. Impact of age-related socio-economic and clinical determinants of quality of life among long-term breast cancer survivors. Maturitas. 2015;81(3):362-70. doi: https://doi.org/10.1016/j.maturitas.2015.03.025
25. Franzoi MA, Rosa DD, Zaffaroni F, et al. Advanced stage at diagnosis and worse clinicopathologic features in young women with breast cancer in Brazil: a subanalysis of the AMAZONA III study (GBECAM 0115). J Glob Oncol. 2019;5:1-10. doi: https://doi.org/10.1200/JGO.19.00263
26. Imran M, Al-Wassia R, Alkhayyat SS, et al. Assessment of quality of life (QoL) in breast cancer patients by using EORTC QLQ-C30 and BR-23 questionnaires: a tertiary care center survey in the western region of Saudi Arabia. PLoS One. 2019;14(7):e0219093. doi: https://doi.org/10.1371/journal.pone.0219093
27. Fobair P, Spiegel D. Concerns about sexuality after breast cancer. Cancer J. 2009;15(1):19-26. doi: https://doi.org/10.1097/PPO.0b013e31819587bb
Recebido em 9/3/2023
Aprovado em 10/5/2023
Executive-Editor: Letícia Casado. Orcid iD: https://orcid.org/0000-0001-5962-8765
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde