ORIGINAL ARTICLE
The Utility of the Liverpool Adverse Drug Reaction Assessment Tools in the Evaluation of Chemotherapy-Induced Nausea and Vomiting in Children
Utilidade das Ferramentas de Avaliação de Reações Adversas a Medicamentos de Liverpool na Análise de Náuseas e Vômitos Induzidos por Quimioterapia em Crianças
La Utilidad de las Herramientas de Evaluación de Reacciones Adversas a Medicamentos de Liverpool en la Evaluación de Náuseas y Vómitos Inducidos por Quimioterapia en Niños
doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n3.3986
Elisangela Costa Lima1; Thais de Barros Fernandes2; Marcelo Gerardin Poirot Land3; Caio Gonzalez4; Colin Thorbinson5; Caroline Bains6; Louise E. Bracken7; Matthew Peak8; Barry Pizer9
1Universidade Federal do Rio de Janeiro (UFRJ), Faculdade de Farmácia. Rio de Janeiro (RJ), Brazil. E-mail: eclima.ufrj@gmail.com. Orcid iD: https://orcid.org/0000-0002-0101-790X
2Fundação Oswaldo Cruz (Fiocruz), Escola Nacional de Saúde Pública Sérgio Arouca (Ensp). Rio de Janeiro (RJ), Brazil. E-mail: fernandes.thaisb@gmail.com. Orcid iD: https://orcid.org/0000-0001-5086-5676
3,4UFRJ, Instituto de Puericultura e Pediatria Martagão Gesteira. Rio de Janeiro (RJ), Brazil. E-mails: land.marcelo@gmail.com; caioenf@hotmail.com. Orcid iD: https://orcid.org/0000-0001-9792-3167; Orcid iD: https://orcid.org/0000-0003-3474-3454
5-9Alder Hey Children’s NHS Foundation Trust, Paediatric Medicines Research Unit. Liverpool, United Kingdom. E-mails: colin.thorbinson@mft.nhs.uk; c.bains1@nhs.net; louise.bracken@alderhey.nhs.uk; matthew.peak@alderhey.nhs.uk; barry.pizer@alderhey.nhs.uk. Orcid iD: https://orcid.org/0000-0002-8987-0349; Orcid iD: https://orcid.org/0009-0005-8418-2722; Orcid iD: https://orcid.org/0000-0002-9632-2252; Orcid iD: https://orcid.org/0000-0003-1909-3211; Orcid iD: https://orcid.org/0000-0003-4945-6386
Corresponding author: Elisangela Costa Lima. UFRJ, Faculdade de Farmácia. Rio de Janeiro (RJ), Brazil. CEP 21941-902. E-mail: eclima.ufrj@gmail.com
RESUMO
Introdução: Náuseas e vômitos induzidos por quimioterapia (NVIQ) são reações adversas a medicamentos (RAM) comuns em crianças em tratamento oncológico. Novas ferramentas de Avaliação de Causalidade e Evitabilidade de RAM de Liverpool (LCAT e LAAT) foram validadas e auxiliam a categorização de fatores de risco. Contudo, até o momento, nenhum estudo comparou a utilidade e os resultados de sua aplicação para NVIQ em países com diferentes níveis de desenvolvimento. Objetivo: Investigar a utilidade da LCAT e LAAT na avaliação de NVIQ. Método: Estudo observacional prospectivo com crianças de 4 a 16 anos do Alder Hey Children's Hospital (Liverpool, Reino Unido) e do Instituto de Puericultura e Pediatria Martagão Gesteira (Rio de Janeiro, Brasil). As crianças (ajudadas pelos pais) preencheram um diário de sintomas durante e até 24 horas após administração da quimioterapia. Informações sobre diagnóstico subjacente, história médica pregressa e medicamentos administrados foram coletadas do prontuário do paciente. Relatos de casos foram preparados e a relação temporal entre náuseas e vômitos e exposição à quimioterapia, incluindo qualquer estratégia para prevenir NVIQ, foi registrada para análise da causalidade e evitabilidade com o auxílio de LCAT e LAAT, respectivamente. Resultados: Houve 26 notificações de NVIQ em 36 ciclos de quimioterapia. A causalidade foi 'definida' para 24 casos. Foram consideradas 'definitivamente evitáveis' 20 RAM e 'não evitáveis', quatro. A seleção de opções terapêuticas inadequadas e a omissão de antieméticos foram os principais problemas evitáveis. Conclusão: O LCAT e LAAT foram úteis para avaliar NVIQ em crianças em dois hospitais diferentes.
Palavras-chave: efeitos colaterais e reações adversas relacionados a medicamentos; náusea; vômito; neoplasias; criança.
ABSTRACT
Introduction: Chemotherapy-induced nausea and vomiting (CINV) are common adverse drug reactions (ADR) experienced by children undergoing treatment for cancer. New paediatric ADR Assessment Causality and Avoidability tools (LCAT and LAAT) of Liverpool are suitable for categorizing factors related to ADR prevention and improving patient care. Still, no studies to date have compared the utility and results of its application for CINV in countries with different levels of development. Objective: To investigate the utility of the Liverpool Adverse Drug Reaction Causality and Avoidability Assessment Tools (LCAT and LAAT) in assessing CINV in children. Method: Prospective observational study of CINV assessment in children aged 4 to 16 years from Alder Hey Children's Hospital (Liverpool, UK) and “Instituto de Puericultura e Pediatria Martagão Gesteira” (Rio de Janeiro, Brazil). Children (helped by the parents) completed a symptom diary during chemotherapy and for 24 hours after treatment. Information regarding underlying diagnosis, past medical history, and medications administered was collected from the patient record. Case reports were prepared, and the temporal relationship between nausea and vomiting and exposure to chemotherapy, including any strategy to prevent CINV, was recorded. The causality and avoidability were assessed with LCAT and LAAT, respectively. Results: There were 26 reports of CINV in 36 chemotherapy cycles. The causality assessment was 'definite' for 24 cases. Twenty ADRs were deemed 'definitely avoidable' and four 'not avoidable'. Selection of inappropriate therapeutic options and non-administration of antiemetic were the most common factors observed in the hospitals studied. Conclusion: The LCAT and LAAT were helpful for assessing CINV in children in two different hospitals.
Key words: drug-related side effects and adverse reactions; nausea; vomiting; neoplasms; child.
RESUMEN
Introducción: Las náuseas y vómitos inducidos por quimioterapia (NVIQ) son reacciones adversas a medicamentos (RAM) comunes en niños en tratamiento oncológico. Nuevas herramientas de Evaluación de Causalidad y Evitabilidad de RAM de Liverpool (LCAT y LAAT) han sido validadas y ayudan en la categorización de factores de riesgo. Sin embargo, ningún estudio ha comparado su utilidad y resultados para evaluación de NVIQ en países con diferentes niveles de desarrollo. Objetivo: Investigar la utilidad de LCAT y LAAT en la evaluación de NVIQ. Método: Estudio observacional prospectivo con niños de 4 a 16 años del Alder Hey Children's Hospital (Liverpool, Reino Unido) y del Instituto de Pediatría Martagão Gesteira (Río de Janeiro, Brasil). Los niños (ayudados por los padres) completaron un diario de síntomas durante y hasta 24 horas después de la quimioterapia. La información sobre el diagnóstico subyacente, la historia médica previa y los medicamentos se recopiló de la historia clínica médico del paciente. Se prepararon informes de casos y se registró la relación temporal entre las RAM y la exposición a la quimioterapia, incluyendo cualquier estrategia para prevenir NVIQ, para análisis de causalidad y evitabilidad con LCAT y LAAT, respectivamente. Resultados: Hubo 26 notificaciones de NVIQ en 36 ciclos de quimioterapia. La causalidad fue "definida" para 24 casos. Fueron consideradas "definitivamente evitables" 20 RAM y "no evitables", cuatro. La selección de opciones terapéuticas inadecuadas y la omisión de antieméticos fueron los principales problemas evitables. Conclusión: LCAT y LAAT fueron útiles para evaluar NVIQ en niños en dos hospitales diferentes.
Palabras clave: efectos colaterales y reacciones adversas relacionados con medicamentos; náusea; vómitos; neoplasias; niño.
Oncology patients may experience several adverse drug reactions (ADRs) during treatment. Chemotherapy-induced nausea and vomiting (CINV) is one of the expected ADRs which can impact the patient’s quality of life, causing dehydration, malnutrition, delays or changes in treatment, increased length of stay and therefore cost of hospitalization1-4. CINV is an example of an ADR that can be avoided with antiemetic drugs such as 5-HT3 receptor antagonists, NK1 antagonists, dopamine blockers, corticosteroids, antipsychotics (levomepromazine), and benzodiazepines2,5. In the last five years, the WHO Collaborating Centre for International Drug Monitoring received more than 5,000 reports of CINV in children6.
Children of different ages undergo a variety of physiological changes with inter patient variability that influence pharmacokinetics7, meaning that the analysis and monitoring of ADRs related to chemotherapy are essential, considering the risk-benefit balance. Therefore, there is a need for methods to help this evaluation process in paediatric cancer care. Two new tools for the characterisation of ADRs in children have been developed and validated: the Liverpool Causality Assessment Tool (LCAT)8 and the Liverpool Avoidability Assessment Tool (LAAT)9 using prospective and retrospective case reports in the United Kingdom (UK).
This pilot study aimed to evaluate the utility of LCAT and LAAT in assessing CINV in paediatric oncology patients in both middle- and high-income settings.
METHOD
A prospective observational study assessed the occurrence of CINV among children in two hospital cohorts: (a) Alder Hey Children's Hospital (AHCH, Liverpool, UK) and (b) Instituto de Puericultura e Pediatria Martagão Gesteira - Paediatric Teaching Hospital (IPPMG, Rio de Janeiro, Brazil). This study took advantage of an existing collaboration between these two hospitals to study the utility of the assessment tools in hospitals with differing CINV prevention and management strategies and in different income settings.
Alder Hey Children's NHS Foundation Trust (AHCH) is a large tertiary paediatric hospital providing general and specialty care for over 275,000 children and young people in the UK each year. It is a leading paediatric research centre with several academic partners, including the University of Liverpool (UoL), which works closely with the Paediatric Medicines Research Unit (PMRU) Alder Hey. AHCH is a National Institute for Health Research (NIHR) Principal Treatment Centre (PTC) for paediatric oncology, providing cancer care to approximately 130 new children and young people per year, and had formal and up to date CINV prevention and management guidelines in use.
“Instituto de Puericultura e Pediatria Martagão Gesteira” (IPPMG) of the “Universidade Federal do Rio de Janeiro” (UFRJ) is a mid-size hospital of the Public Health Brazilian System that provides complex care. IPPMG is a site for outreach activities and undergraduate and post-graduate education (medical residency and multi-professional residency in child and adolescent health). Research on medicines use in IPPMG is carried out in collaboration with the “Observatório de Vigilância e Uso de Medicamentos” of UFRJ. IPPMG provides cancer care to around 70 new children annually and unlike AHCH had no formal CINV management guidelines.
In both hospitals, a team of pharmacists work closely with other staff to support the chemotherapy administration, perform adherence monitoring and provide patient and staff education.
The study included children and young people aged 4 to 16 years of age on the Oncology Unit (day-case and inpatients) receiving intravenous chemotherapy treatment at AHCH or IPPMG. This age range was chosen in respect of children and young people’s capacity for ADR description and reporting10.
Inpatient and day-case patients (outpatients) were identified daily for three months (AHCH: between August to October 2017 and at IPPMG: March to May 2018) by examining on the electronic hospital record systems. Children and young people who met the inclusion criteria were invited to take part in the study. Informed consent was obtained from the parent/legal guardian or young person, and written assent was obtained for children over six years of age who wished to participate in the study. Patients were eligible to be included in the study more than once and each chemotherapy cycle was considered a new case.
The occurrence of CINV varies between chemotherapy regimens and is dependent upon the emetogenic potential of individual and combinations of the chemotherapy drugs employed11. The emetogenic classification framework is based on the incidence of emesis in the absence of prophylaxis; categorised as high or very high (more than 90% of incidence), moderate (30% to 90% of incidence), low (10% to 30% of incidence) and minimum (less than 10% of incidence)12.
Drug administration data were collected from the hospital electronic prescribing systems and chemotherapy scheduling by the Pharmacy Services at both hospitals.
Exclusion criteria included chemotherapy of low emetogenic potential11,12, difficulty in understanding study terms/concepts (such as nausea), or problems in using the diary for data collection.
Episodes of vomiting, retching, or reports of nausea were considered as suspected CINV cases2. A vomiting episode was considered the expulsion of any stomach contents by the mouth and retching as an attempt to vomit that was not productive. The acute phase was defined as the time from administration of the first dose of chemotherapy and continuing for 24 hours after administration of the last dose of the chemotherapy cycle13.
Symptom diaries for daily self-completion (children aged from 10 to 16) or supervised-or parent-completed (children aged from 4 to 9) were developed to record any symptoms of CINV. In addition to patient information and treatment, the diaries contained one page for each day of treatment with (i) the following dichotomous or quantitative questions: “Did you feel sick today?”, “Did your nausea and vomiting stop you from eating today?”, “How many times did you vomit/retch today?” and (ii) visual analogue scale (VAS) for assessing the intensity of nausea14,15.
Participants were asked to report any CINV symptoms during their chemotherapy treatment and for 24 hours after administration of the last dose. Diaries completed in the outpatient setting were returned in person to a study investigator, or a copy was emailed to the study team by the parents. The same researcher followed up locally with all children included in both hospitals during data collection. Where CINV was suspected or reported, a pharmacist prepared a case report based on the information needed for ADR assessment, collected from diaries, and the evaluation of patient records16. Each case report was organized in the following data sections: (a) patient characterization (age, gender, health, underlying diseases, and treatment history), (b) prescription drugs and dose administered, emetogenic risk of chemotherapy regimen12, any strategy to prevent and monitor acute nausea and vomiting including antiemetics, known history of hypersensitivity or similar previous reactions and (c) temporal relationship between nausea and vomiting and exposure to chemotherapy agents8,9,11,12.
Each nausea and vomiting episode was investigated. The history of CINV in previous cycles was also recorded in the ADR case report. The LCAT was used to assess causality for every suspected CINV case report, and cases were categorised as unlikely, possible, probable, or definite. Possible, probable, or definite cases were then assessed for avoidability using the LAAT and classified as unassessable, not avoidable, possibly avoidable, or definitely avoidable8,9. LCAT and LAAT are presented in Supplementary Material.
The avoidability assessment considered the patient history, CINV prevention guidelines from AHCH and IPPMG, other updated information sources (good practices to CINV management from literature)2,13,14, as outlined in LAAT glossary9.
The CINV prevention guideline was determined by the chemotherapy regimens emetogenic potential (AHCH) in the UK. The unit has had a guideline for the prevention and management of chemotherapy-induced nausea and vomiting in place since 2001. For regimens of high and very high emetogenic potential, the use of ondansetron and levomepromazine are recommended first line with the addition of dexamethasone if necessary. Infusion of levomepromazine, lorazepam, or aprepitant (for patients over 12 years old) were also considered. In moderate emetogenic potential chemotherapeutic regimens, regular use of ondansetron was recommended with levomepromazine or dexamethasone, if necessary.
At the Brazilian hospital (IPPMG), CINV prevention and management plans were based on the administration of ondansetron up to one hour before chemotherapy administration, maintaining regular oral use for outpatients in the case of CINV. If CINV persisted, the guideline indicated increased frequency and the addition of bromopride in cases of treatment failure. This strategy was discussed and approved by the medical team verbally in a meeting but is not published as a clinical guideline at IPPMG.
Each case report was assessed with LCAT and LAAT by three independent reviewers (a pharmacist, paediatrician, and a nurse) in both hospitals. The results reported were based on the consensus agreement between reviewers. Where no consensus was reached, cases were referred to a panel of two senior investigators (paediatric oncologist and pharmacist) for review, and a panel decision was recorded17. Training on the use of LCAT and LAAT was provided to the reviewers in advance.
Ethical approval for the UK study was obtained from an NHS Research Ethics Committees (REC Reference 17/EM/0300) and in Brazil from an IPPMG/UFRJ Research Ethics Committee (Number: 3.264.238) CAAE (submission for ethical review) 08802019.9.0000.5264 in compliance with Resolution 466/1218 of the National Health Council for research with human beings.
RESULTS
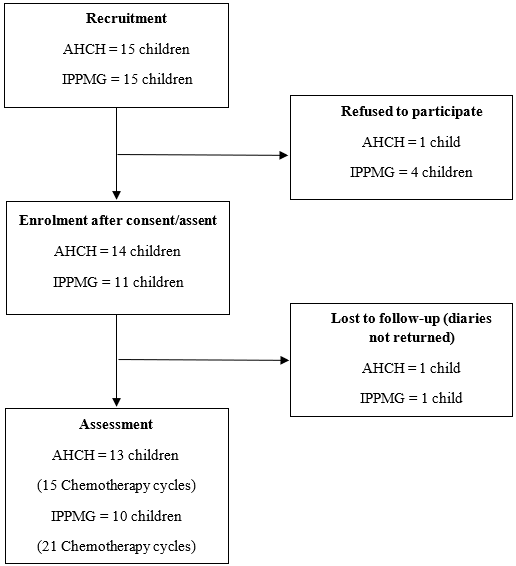
Data from 15 chemotherapy cycles of 13 patients from AHCH (UK) and 21 cycles of 10 patients from IPPMG/UFRJ (Brazil) were collected. Each new cycle of chemotherapy was considered as a new case, totaling 36 cases in both hospitals. Diaries were returned for 36 cases in the UK and Brazil. Two patients (one in UK and one in Brazil) were excluded from study because diaries were not returned (Figure 1, Table 1).
|
|
|
Figure 1. Recruitment and Enrolment Patients |
|
Table 1. Characterization of patients included by number of chemotherapy cycles assessed (n=36) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Captions: AHCH = Alder Hey Children's NHS Foundation Trust, Liverpool, UK; IPPMG = Instituto de Puericultura e Pediatria Martagão Gesteira, Rio de Janeiro, Brazil. Note: Each chemotherapy cycle was considered a new case for assessment. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Overall, the frequency of suspected CINV cases observed at both centres was high but slightly higher in IPPMG (76% versus 67%), However, the chemotherapy regimens at IPPMG were of relatively lower emetogenic potential (moderate versus high) (Table 2).
Table 2. CINV case reports assessment (n=26)
|
CINV case reports |
|
AHCH |
IPPMG |
Total (n) |
|
Causality Assessment |
Definite |
8 |
16 |
24 |
|
|
Probable |
2 |
0 |
2 |
|
Avoidability Assessment |
Definitely avoidable |
4 |
16 |
20 |
|
|
Possibly avoidable |
0 |
0 |
0 |
|
|
Not avoidable |
6 |
0 |
6 |
|
Total |
|
10 |
16 |
26 |
|
Captions: AHCH = Alder Hey Children's NHS Foundation Trust, Liverpool, UK; IPPMG = Instituto de Puericultura e Pediatria Martagão Gesteira, Rio de Janeiro, Brazil. |
||||
The median (interquartile range) of time for assessment using the tools was 2.5 (2.0 – 3.7) and 3 (2.2 – 4.0) minutes for causality and avoidability, respectively.
Among the ten CINV cases observed at AHCH, eight were categorised as ‘definite’ in terms of causality assessment and two as ‘probable’ due to the presence of other factors related to the patient's clinical condition, such as concomitant fever at the time of the reaction. Consensus agreement was reached for eight cases by the three reviewers, two were referred to senior investigator panel for a decision. At IPPMG, the three reviewers reached consensus agreement for all 16 cases which were categorised as definite.
In terms of the avoidability assessments six cases were categorised as ‘not avoidable’, and four as ‘definitely avoidable’ at AHCH. Two cases were referred to the senior investigator panel for a decision. All cases of CINV at IPPMG were categorised as ‘definitely avoidable’ by three reviewers; because, despite the informal clinical guidelines recommended for CINV prevention or treatment, these were not followed or were considered inappropriate according to the strategies available in the literature (for treatment regimens with high emetogenic potential). The guidelines recommended at IPPMG did not specify different doses, frequency of use, or the association of antiemetics of greater effectiveness in cases of higher risk of CINV (greater emetogenic potential).
Both hospitals identified cases which were ‘definitely avoidable’ due to the team not following the dosage or frequency of administering the recommended antiemetics (Table 3).
|
Table 3. Description of problems found in CINV avoidable cases |
|||
|
Inadequate practice |
Number of cases by setting |
|
|
|
|
IPPMG |
AHCH |
|
|
Lack of appropriate therapeutic options with better evidence of effectiveness for CINV in children* |
14 |
None |
|
|
Prophylactic antiemetic not administered (ondansetron) |
5 |
None |
|
|
Antiemetic administered less frequently than recommended (ondansetron) |
4 |
1 |
|
|
Antiemetic available but not administered (bromopride) |
5 |
None |
|
|
Antiemetic administered at inappropriate time (ondansetron) |
3 |
None |
|
|
Antiemetic administered in lower dose than recommended (levomepromazine) |
None |
3 |
|
|
Antiemetic administered in different route from recommended (levomepromazine) |
None |
1 |
|
|
Captions: AHCH = Alder Hey Children's NHS Foundation Trust, Liverpool, UK; IPPMG = Instituto de Puericultura e Pediatria Martagão Gesteira, Rio de Janeiro, Brazil (*) Informal recommendation suggested dimenhydrinate but this drug was not available and bromopride was prescribed in study period. Note: Some cases have more than one problem related to CINV prevention. |
|||
At AHCH, there was a case classed as ‘not avoidable’ where an 11-year-old child who was aware of preventative drugs available for CINV but refused treatment. This was deemed not avoidable as the patient chose not to have any antiemetics rather than the team failing to administer treatment.
DISCUSSION
In this study, 36 chemotherapy cycles in children (4-16 years old) were analysed, and the incidence of CINV was reported in 24 cycles (cases) (72.2%). Despite clinical practical guidelines existence for this population, an overall low rate of guideline concordant care with prophylaxis and management of CINV is presented in literature19.
In terms of causality assessment, the majority of CINV cases were considered as definite (92.3%) with LCAT application, which was expected. Pharmacoepidemiology studies commonly characterize causality as “possible” or “probable”9,20 using other tools. However, the LCAT classification approach is based on binary decisions while the Naranjo tool, (Adverse Drug Reaction Probability Scale) for example, assigns weighted scoring, and has two extra criteria as use of placebo and detection of drug in toxic concentration, inapplicable in most the contexts of cancer care making it difficult to reach a definite causality8.
The verification of the causal link between chemotherapy and the event was favored by the fact that most cases did not involve other health conditions that could cause or aggravate nausea and vomiting. Brain tumors, abdominal and pancreatic tumors, infections, gastritis, gastroenteritis, intestinal obstruction, constipation, and severe pain or exposure to radiotherapy would be examples of alternative causes for nausea and vomiting in children (NICE, Mayo Foundation, NHS and American Cancer Society) that should be considered in CINV assessment. Furthermore, in the complex cases regarding underlying diseases and causes, objective evidence of likely ADR mechanism (dose-dependent relationship chemotherapy with nausea and vomiting) might be considered valuable in attributing causality8.
Assessment of a set of cases with the same type of ADR and the availability of patient record facilitated the rapid application of the assessment tool, making it easy to apply. Another aspect that favored the causality assessment with LCAT was the occurrence of events following the administration of at least one dose of chemotherapy. In the majority of cases, children had a previous history of CINV with the same medications or experienced CINV throughout the course with repeated administration chemotherapy (positive rechallenge). Cases deemed probable or definite warrant further investigation or follow up by healthcare professionals.
In terms of avoidability, all cases in the Brazilian hospital were classified as avoidable. Despite the existence of guidelines for prevention and management in both institutions (Table 2), the antiemetic therapy used was considerably different from CINV evidence-based practice in the IPPMG. The use of 5-HT3 antagonists (ondansetron or granisetron) with dexamethasone (when possible), or aprepitant, levomepromazine, chlorpromazine, and nabilone is recommended for children using highly emetogenic chemotherapy2,14,21.
Another factor observed was the timing of administration of ondansetron (30 minutes to one hour before chemotherapy) and regular maintenance (every six to eight hours) for at least the first 24 hours after chemotherapy2,14,21. This practice was recommended in both hospitals (on formal guideline at AHCH and informal guideline at IPPMG). However, it was not followed in some of the cases considered definitively avoidable in both IPPMG and AHCH.
ADRs reported in oncology tend to be underestimated and are not always related to clinical studies22. Therefore it is important for healthcare professionals, primarily the pharmacist, to actively undertake pharmacovigilance surveillance using tools that provide an objective assessment for an assertive care process to identify relevant risks and quick decision making23. It has been shown that the use of the LCAT and LAAT has a practical application in this process.
Analysis of these reactions can (i) give the individual team information for monitoring CINV for each child and (ii) increase the current knowledge base (providing evidence of incidence of ADR, for example). Adherence to clinical practice guidelines, including those related to ADRs, should be measured as one quality dimension of universal health coverage24.
However, full adherence to the guidelines was not expected. A Brazilian study25 evaluating adherence to CINV prevention guidelines in adults treated in a private hospital identified a lack of compliance in 78% of reported cases. The authors indicated that treatments (i) of hematological tumors, (ii) with high emetogenic potential, or (iii) with two or more chemotherapeutic agents were associated with the highest rate of adherence by professionals25.
The LCAT and LAAT were useful to define causality of CINV cases and categorize factors related to prevention of these reactions respectively, including use of supportive therapies, which may encourage the translation of best practices into health services, particularly regarding adherence to guidelines, pharmacovigilance training, and drug selection in a Brazilian and a British hospital. The possibility of leading the team to reflect on best practices, based on a faceted analysis of the causality and avoidability of events, is one of the most relevant aspects of the use of these tools. Both hospitals provided a clinical pharmacy service but without specific identification strategies for CINV and other ADRs in oncology.
Concepts and practices for pharmacovigilance and patient safety remain a low priority, especially in developing countries like Brazil26. The real-life data obtained from this research showed that the LCAT and LAAT application was beneficial for CINV assessment in pediatric cancer patients and could have a broader application in assessing ADRs in children.
The use of diaries for this pilot was considered a good strategy for data collection and had high compliance, with more than 90% of patients enrolled returning their completed diaries.
As this was a pilot study, the main limitation was the small number of patients included and information bias. It is believed that the use of CINV symptom diaries and the local availability of the same researcher for data collection in both hospitals minimized the risk of introducing this bias. Therefore, a new larger study is recommended.
The study pointed to the utility of the tool and the need for further research on the prevalence and impact of CINV on children, as the findings indicate there is potential to reduce the occurrence and impact of CINV in children. Although both validation studies included oncologic cases, more research is needed to increase knowledge in this field, especially regarding the possibility of preventing reactions in developing countries in which pharmacovigilance systems are not yet robust10.
CONCLUSION
This study found that the application of LCAT and LAAT was helpful to assess CINV cases to healthcare professionals to try and mitigate the risk in subsequent cycles. The CINV assessment in the two hospital pediatric cohorts (in UK and Brazil) with LCAT and LAAT highlighted the high frequency of these ADRs (67% and 76%, respectively). According to the current evidence base, almost 80% of CINV cases were considered avoidable since they did not use the best clinical practices: dose, frequency, time of administration, and inappropriate selection of antiemetic regimen under the emetogenic potential of chemotherapy and age or weight of the child. Therefore, indicating that there is potential to avoid or reduce CINV in a large proportion of cases.
The findings from this pilot study suggest that applying the new Liverpool Tools could help the healthcare team monitor its practices to avoid ADRs in children and improve cancer care in both middle and high-income countries. A thorough exploration of avoidable ADR cases could inform the development of practical interventions that can be translated into clinical practice.
CONTRIBUTIONS
Elisangela Costa Lima, Caroline Bains, Louise E. Bracken, Barry Pizer and Matthew Peak contributed to the design and methodology. Thais de Barros Fernandes, Marcelo Gerardin Poirot Land, Caio Gonzalez, Caroline Bains and Colin Thorbinson contributed to the investigation and formal analysis. Elisangela Costa Lima, writing of the original draft. Louise E. Bracken, Barry Pizer and Matthew Peak, writing, review and editing. All authors approved the final manuscript for publication.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
FUNDING SOURCES
National Council for Scientific and Technological Development (CNPq) (Grant number: 421992/2016-6).
REFERENCES
1. Van Laar ES, Desai JM, Jatoi A. Professional educational needs for chemotherapy-induced nausea and vomiting (CINV): multinational survey results from 2388 health care providers. Support Care Cancer. 2015;23(1):151-7. doi: https://doi.org/10.1007/s00520-014-2325-x
2. Phillips RS, Friend AJ, Gibson F, et al. Antiemetic medication for prevention and treatment of chemotherapy-induced nausea and vomiting in childhood. Cochrane Database of Syst Rev. 2016;2(2):CD007786. doi: https://doi.org/10.1002/14651858.CD007786.pub3
3. Patel H, Gurumurthy P. Improving medication safety in oncology care: impact of clinical pharmacy interventions on optimizing patient safety. Int J Clin Pharm. 2019;41(4):981-92. doi: https://doi.org/10.1007/s11096-019-00860-0
4. Evans A, Vingelen MB, Yu C, et al. Nausea in numbers: electronic medical record nausea and vomiting assessment for children with cancer. J Pediatr Oncol Nurs. 2020;37(3):195-203. doi: https://doi.org/10.1177/1043454219900467
5. Antonarakis ES. Nausea and vomiting associated with cancer chemotherapy: drug management in theory and in practice. Arch Dis Child. 2004;89(9):877-80. doi: https://doi.org/10.1136/adc.2003.037341
6. Uppsala Monitoring Centre [Internet]. Sweden: Uppsala Monitoring Centre; [cited 2023 Mar 23]. Available from: https://who-umc.org/
7. Anderson BJ, Lerman J, Coté CJ. Pharmacokinetics and Pharmacology of drugs used in children. In: Coté CJ, Lerman J, Anderson BJ, editors. A practice of anesthesia for infants and children [Internet]. Philadelphia (PA): Elsevier; 2019 [cited 2023 Mar 23]. p. 100-176.e45. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780323429740000070
8. Gallagher RM, Kirkham JJ, Mason JR, et al. Development and inter-rater reliability of the Liverpool adverse drug reaction causality assessment tool. PLoS One. 2011;6(12):e28096. doi: https://doi.org/10.1371/journal.pone.0028096
9. Bracken LE, Nunn AJ, Kirkham JJ, et al. Development of the Liverpool adverse drug reaction avoidability assessment tool. PLoS One. 2017;12(1):e0169393. doi: https://doi.org/10.1371/journal.pone.0169393
10. Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol. 2015;8(4):449-60. doi: https://doi.org/10.1586/17512433.2015.1053391
11. Aapro M, Jordan K, Feyer P. Prevention of nausea and vomiting in cancer patients [Internet]. London: Springer Healthcare; c2013 [cited 2023 Mar 23]. Available from: https://link.springer.com/book/10.1007/978-1-907673-58-0
12. Paw Cho Sing E, Robinson PD, Flank J, et al. Classification of the acute emetogenicity of chemotherapy in pediatric patients: a clinical practice guideline. Pediatr Blood Cancer. 2019;66(5):e27646. doi: https://doi.org/10.1002/pbc.27646
13. Patel P, Robinson PD, Thackray J, et al. Guideline for the prevention of acute chemotherapy-induced nausea and vomiting in pediatric cancer patients: a focused update. Pediatr Blood Cancer. 2017;64(10):e26542. doi: https://doi.org/10.1002/pbc.26542
14. Dupuis LL, Sung L, Molassiotis A, et al. 2016 updated MASCC/ESMO consensus recommendations: Prevention of acute chemotherapy-induced nausea and vomiting in children. Support Care Cancer. 2017;25(1):323-31. doi: https://doi.org/10.1007/s00520-016-3384-y
15. Mazlum S, Chaharsoughi NT, Banihashem A, et al. The effect of massage therapy on chemotherapy-induced nausea and vomiting in pediatric cancer. Iran J Nurs Midwifery Res. 2013;18(4):280-4. Cited in: PubMed; PMID: 24403922.
16. Smyth RL, Peak M, Turner MA, et al. ADRIC: Adverse Drug Reactions In Children – a programme of research using mixed methods. Programme Grants Appl Res. 2014;2(3):1-184. doi: https://doi.org/10.3310/pgfar02030
17. Thiesen S, Conroy EJ, Bellis JR, et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children - a prospective observational cohort study of 6,601 admissions. BMC Med. 2013;11:237. doi: https://doi.org/10.1186/1741-7015-11-237
18. Conselho Nacional de Saúde (BR). Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília, DF. 2013 jun 13; Seção 1:59.
19. Beauchemin M, Sung L, Hershman DL, et al. Guideline concordant care for prevention of acute chemotherapy-induced nausea and vomiting in children, adolescents, and young adults. Support Care Cancer. 2020;28(10):4761-9. doi: https://doi.org/10.1007/s00520-020-05310-6
20. Bellare PS, Ashwin K, Prakash PU S, et al. A retrospective evaluation of adverse drug reactions due to cancer chemotherapy in a tertiary care hospital in south India. J Young Pharm. 2016;8(3):251-4. doi: https://doi.org/10.5530/jyp.2016.3.14
21. Children’s Cancer and Leukaemia Group [Internet]. Leicester (UK): CCLG; [date unknown]. Chemotherapy induced nausea and vomiting; [cited 2021 Dec 2]. Available from: https://www.cclg.org.uk/CSOIR/Chemotherapy-induced-nausea-and-vomiting
22. Fornasier G, Taborelli M, Francescon S, et al. Targeted therapies and adverse drug reactions in oncology: the role of clinical pharmacist in pharmacovigilance. Int J Clin Pharm. 2018;40(4):795-802. doi: https://doi.org/10.1007/s11096-018-0653-5
23. Abunahlah N, Sancar M, Dane F, et al. Impact of adherence to antiemetic guidelines on the incidence of chemotherapy-induced nausea and vomiting and quality of life. Int J Clin Pharm. 2016;38(6):1464-76. doi: https://doi.org/10.1007/s11096-016-0393-3
24. Organisation for Economic Cooperation and Development; World Health Organization, World Bank Group. Delivering quality health services: a global imperative. Geneva: WHO; 2018.
25. França MS, Usón Junior PLS, Antunes YPPV, et al. Assessment of adherence to the guidelines for the management of nausea and vomiting induced by chemotherapy. Einstein (São Paulo). 2015;13(2):221-5. doi: https://doi.org/10.1590/S1679-45082015AO3097
26. Elshafie S, Zaghloul I, Roberti AM. Pharmacovigilance in developing countries (part I): importance and challenges. Int J Clin Pharm. 2018;40(4):758-63. doi: https://doi.org/10.1007/s11096-017-0570-z
Recebido em 3/5/2023
Aprovado em 7/7/2023
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde