ORIGINAL ARTICLE
Bioequivalence/bioavailability Study of a New Formulation of Imatinib Mesylate 100 mg Coated Tablets versus Glivec® in Brazilian Healthy Male Volunteers in Fed State
Estudo de Bioequivalência/Biodisponibilidade de uma Nova Formulação de Mesilato de Imatinibe versus Glivec® em Voluntários Saudáveis Brasileiros em Condições Alimentares
Estudio de Bioequivalencia/Biodisponibilidad de una Nueva Formulación de Mesilato de Imatinib versus Glivec® en Voluntarios Saludables Brasileños Bajo Condiciones Alimentarias
https://doi.org/10.32635/2176-9745.RBC.2023v69n4.4060
Carlos Eduardo Sverdloff1; Vinicius Marcondes Rezende2; Paulo Alexandre Rebelo Galvinas3; Guilherme Araújo Pinto4; Lygia Nerath Bonanato5; Fernando Bastos Canton Pacheco6
1,2ATCgen Pesquisa e Desenvolvimento à Saúde Ltda. Campinas (SP), Brazil. E-mails: carsver@atcgen.com.br; vinicius@atcgen.com.br. Orcid iD: https://orcid.org/0000-0001-8676-5431; Orcid iD: https://orcid.org/0000-0002-6586-0496
3,4Magabi Pesquisas Clínicas e Farmacêuticas. Itapevi (SP), Brazil. E-mails: paulo.galvinas@magabi.com.br; guilherme.pinto@eurofarma.com. Orcid iD: https://orcid.org/0000-0002-1798-4355; Orcid iD: https://orcid.org/0000-0003-2407-9211
5Eurofarma Laboratórios. Itapevi (SP), Brazil. E-mail lygia.bonanato@eurofarma.com. Orcid iD: https://orcid.org/0000-0002-3007-6343
6Centro Avançado de Estudo e Pesquisa (Caep). Campinas (SP), Brazil. E-mail: fernando.pacheco@synvia.com. Orcid iD: https://orcid.org/0000-0001-9383-3244
Corresponding author: Carlos Eduardo Sverdloff. Rua Barão de Anhumas, 152, apto. 54 – Bosque. Campinas (SP), Brazil. CEP 13026-020. E-mail: carsver@atcgen.com.br
ABSTRACT
Introduction: Imatinib mesylate is currently the first-line oral treatment for all stages of chronic myeloid leukemia (CML) and is also used in some cases of gastrointestinal stromal tumor (GIST) and acute lymphoblastic leukemia (ALL). Objective: Investigate the bioavailability of two products containing imatinib mesylate, 100 mg coated tablet, to determine if they are bioequivalent. Method: The study was conducted using an open-label, randomized, balanced design and the formulations were administered orally in a single dose to 48 healthy adult males, in fed state, followed by sequential blood withdraws for the next 72 hours. Forty-eight male healthy volunteers were selected to participate in the study. Test formulation from Eurofarma Laboratórios S.A. Brazil was compared to from Novartis Biociências S.A. The comparative bioavailability of the formulations was assessed based on statistical comparisons of relevant pharmacokinetic parameters obtained from drug concentration data from collected blood samples measured using an analytical method based on high-performance liquid chromatography coupled to mass spectrometry. Results: The ratio of the geometric means between the test and the reference, with a 90% confidence interval, of pharmacokinetic parameters for Cmax was 102.26% (94.17-111.04%) and for AUC0-t was 101.24% (95.19-107.68%). Conclusion: Imatinib mesylate 100 mg (test product) from Eurofarma Laboratórios S.A. was considered bioequivalent to the reference Glivec® 100 mg manufactured by Novartis Biociências S.A, and the test product can be interchangeable with the reference, based on their pharmacokinetic performance.
Key words: imatinib mesylate; therapeutic equivalency; tandem mass spectrometry; tyrosine protein kinase inhibitors.
RESUMO
Introdução: O mesilato de imatinibe é atualmente o tratamento oral de primeira linha para todos os estágios de leucemia mieloide crônica (LMC) e é usado também em alguns casos de tumores gastrointestinais (GIST) e na leucemia linfoide aguda (LLA). Objetivo: Investigar a biodisponibilidade de dois produtos contendo mesilato de imatinibe de 100 mg em comprimidos revestidos para determinar se são bioequivalentes. Método: Estudo conduzido usando um desenho aberto, randomizado e balanceado. As formulações foram administradas de forma oral em única dose a 48 participantes saudáveis do sexo masculino após alimentação, seguido de coletas de sangue por 72 horas. Quarenta e oito participantes saudáveis foram selecionados para participar do estudo. A formulação teste da Eurofarma Laboratórios S.A. foi comparada com a formulação referência da Novartis Biociências S.A. A biodisponibilidade relativa das formulações foi avaliada em comparações estatísticas dos parâmetros farmacocinéticos relevantes obtidos de concentrações de droga das amostras coletadas mediante a utilização de um método analítico baseado em cromatografia líquida de alta performance acoplada à espectrometria de massas. Resultados: A razão das médias geométricas entre teste e referência com intervalo de confiança 90% dos parâmetros farmacocinéticos para Cmáx foi de 102,26% (94,17-111,04%) e para ASC0-t foi de 101,24% (95,19-107,68). Conclusão: Mesilato de imatinib 100 mg (produto teste) da Eurofarma Laboratórios S.A. foi considerado bioequivalente ao Glivec® 100 mg produzido por Novartis Biociências S.A., e o produto teste pode ser intercambiável como referência com base em seu desempenho farmacocinético.
Palavras-chave: mesilato de imatinib; equivalência terapêutica; espectrometria de massas em tandem; inibidores de proteína tirosina quinase.
RESUMEN
Introducción: El mesilato de imatinibe es actualmente el tratamiento oral de primera línea para todos los estadios de leucemia mieloide crónica (LMC) y es usado también en algunos casos de tumores gastrointestinales (GIST) y leucemia linfoide aguda (LLA). Objetivo: Investigar la biodisponibilidad de dos productos de mesilato de imatinibe de 100 mg en comprimidos revestidos para determinar si son bioequivalentes. Método: Estudio ejecutado usando un diseño abierto, aleatorizado y balanceado. Las formulaciones fueron administradas de forma oral en dosis única a 48 participantes saludables de sexo masculino en condiciones de alimentación, seguidas de muestras de sangre por 72 horas. Cuarenta y ocho participantes saludables fueron seleccionados para participar del estudio. La formulación de prueba de Eurofarma Laboratórios S.A. fue comparada con la formulación referencia de Novartis Biociências S.A. La biodisponibilidad relativa de las formulaciones fue evaluada mediante comparaciones estadísticas de los parámetros farmacocinéticos relevantes obtenidos de concentraciones del fármaco de muestras recolectadas con la utilización de un método analítico basado en cromatografía de alto rendimiento acoplada a espectrometría de masas. Resultados: La relación de las medias geométricas entre la prueba y la referencia con un intervalo de confianza del 90% de los parámetros farmacocinéticos para Cmax fue 102,26% (94,17-111,04%) y para AUC0-t fue 101,24% (95,19-107,68). Conclusión: El mesilato de imatinib 100 mg (producto de prueba) de Eurofarma Laboratórios S.A. fue considerado bioequivalente al Glivec® 100 mg producido por Novartis Biociências S.A. y el producto de prueba puede ser intercambiable con el de referencia en función de su desempeño farmacocinético.
Palabras clave: mesilato de imatinib; equivalencia terapéutica; espectrometría de masas en tándem; inhibidores de la tirosina proteína quinasa.
INTRODUCTION
Chronic myeloid leukemia (CML), a myeloproliferative disease characterized by the clonal expansion of hematopoietic cells that contain the Philadelphia chromosome (also called BCR-ABL), the result of a reciprocal translocation between the long arms of chromosomes 9 (BCR) and 22(ABL), forming the oncogene BCR-ABL t(9: 22)(q34;q11). Imatinib mesylate, approved in the United States in 2001, is a drug whose development has made it possible, for the vast majority of patients, to orally treat and control avoiding bone marrow transplantation. Imatinib is a competitive inhibitor of tyrosine kinase, being effective against CML1.
Imatinib mesylate is known as the drug of choice and it is presently the first-line treatment for all phases of CML2-5 and is the first-line treatment for CML patients treated at SUS, Brazil’s National Health System, but 4 tyrosine kinase inhibitors are currently approved for use in first line setting (imatinib, dasatinib, nilotinib and bosutinib). Overall survival of about 93% in 8 years has dramatically changed the prognostic compared to that alpha-interferon since early 2001 when imatinib was approved by the Food and Drug Administration FDA2-6. Imatinib is an oral protein tyrosine kinase inhibitor which inhibits (competitively) tyrosine kinase BCR-ABL platelet-derived growth factor receptors (PDGFRs) and c-KIT receptors for stem cell factor (SCF). Constitutive activation of these tyrosine kinases is crucial to the pathogenesis of certain tumors and myeloproliferative disorders, regulating the expression of the genes associated. In colony formation assays, where ex vivo bone marrow and peripheral blood samples are used, imatinib shows inhibition of BCR-ABL-positive colonies from patients with CML. In vivo, imatinib inhibits tumor growth of BCR-ABL-transfected murine myeloid cells, as well as BCR-ABL-positive leukemic cell lines derived from CML patients in blast crisis7,8.
Imatinib is also an inhibitor of PDGF receptor tyrosine kinase and SCF and inhibits PDGF and SCF-mediated cellular events. In vitro, imatinib inhibits proliferation and induces apoptosis in gastrointestinal stromal tumor (GIST) cells, which have a mutation that leads to activation of the c-Kit gene. Imatinib is a competitive antagonist for the Adenosine Tri-Phosphate (ATP) binding site, thereby blocking the ability of c-Kit to transfer phosphate groups from ATP to protein tyrosine residues, disrupting c-Kit-mediated signal transduction. In CML, the process is similar, competitively inhibiting the binding of ATP with the tyrosine kinase domain of ABL, preventing the phosphorylation of substrates, thus blocking the activity of BCR-ABL and inducing leukemic cell death8.
Imatinib mesylate is the first-line CML therapy, and it is also used in the acute lymphoblastic leukemia (ALL) that are Philadelphia chromosome-positive (Ph+), some types of GIST, hyper eosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis, and myelodysplastic syndrome9.
The bioavailability of imatinib is around 98% irrespective of dosage form (solution, capsule, or tablet) or dosage (100 or 400 mg) and the absorption is not significantly affected by food. Imatinib is rapidly absorbed after oral administration with a peak plasma concentration occurring at about 3 hours (1.5-5h)9-12.
The drug accumulation in blood is 1.5-3 fold at steady state with multiple dose administration on a once daily schedule, compared with concentrations observed after the first imatinib dose and gender has no effect on the pharmacokinetics of imatinib13.
Imatinib mesylate is well-absorbed by the gastrointestinal tract, with a very high bioavailability, reaching concentrations between 1,000 to about 1,300 ng/mL at trough level (minimum level at steady state)14,15. At clinically relevant concentrations, imatinib mesylate is highly bound to plasma protein, mainly to albumin and to alpha1-acid glycoprotein16. Imatinib undergoes a considerable metabolism in the liver, mainly via CYP3A4. The main metabolite, CGP 74588 (desmethyl-imatinib), is known to be active, and the elimination of this and other metabolites is more than 90% through the bile. The elimination half-life of imatinib mesylate is approximately 18 h, with a range from 14 to 23 hours, with two-to three-fold accumulation at steady-state17. When investigated in healthy adult humans after one week from ingestion of a single dose of 14C-radiolabelled imatinib, 80% of the dose had been excreted; the predominant mode of elimination was fecal (67%) and the less significant is via urine (13%)10,18-20.
Efficacy and safety of the reference product Glivec® have already been proven in several clinical trials11,17,21-23.. The current trial was conducted at “Centro Avançado de Estudo e Pesquisa (CAEP)”, Campinas, São Paulo, Brazil.
METHOD
The test product was 100 mg imatinib mesylate coated tablet developed by Eurofarma Laboratórios S.A., and following Anvisa’s recommendations, and the reference product was Glivec®, 100 mg imatinib coated tablet, manufactured and distributed by Novartis Biociências S.A.
Forty-eight healthy male volunteers between 18 and 49 years, having body mass index (BMI) between 18.70 kg/m² and 29.99 kg/m² were enrolled according to the inclusion and exclusion criteria. Volunteers were subject to laboratory clinical evaluation including hemogram, hepatic and kidney function, blood lipidic profile, cardiovascular assessment and general clinical evaluation.
The sample size was estimated by statistical methods considering a power of 90% for the range of the acceptance criteria of bioequivalence (80-125%) with an intra-subject coefficient of variability of 20%. The estimated sample size was 48 subjects, considering a 20% of dropout. Of the initial 48 volunteers enrolled, only 42 ended the trial.
Samples from each one of the 42 subjects were sent to the analytical facility, and the data generated from these samples were sent to the statistician.
The volunteers were confined the day before administration and remained fasted at least eight hours before and four hours after the drug administration. All volunteers received a coated tablet containing 100 mg of imatinib, administered orally in a single dose, 30 minutes after standardized breakfast, with 200 mL of water. Fluid (water) consumption was allowed up to two hours before drug administration and two hours after administration. Drug administrations were performed around 7:00 am according to the randomization list.
The subjects were lectured about the study details and all their questions were clarified to facilitate an informed decision on whether to join the study or not. Those who accepted to participate signed the Informed Consent Form. The Institutional Review Board (IRB) of “Faculdade de Ciências Médicas da Universidade Estadual de Campinas (Unicamp)” approved the study, CAAE (submission for ethical review) 0167.0.146.000-10. The study followed the Good Clinical Practices guidelines, in compliance with Resolution 466/201224 of the National Health Council.
This study was single-center, open-label, randomized, crossover 2-periods, with 2 treatments, Test (T) and Reference (R), being balanced by two (2) treatment sequences (RT and TR – test/reference and reference/test). After screening, the formulations were administered as a single dose orally followed by blood sampling for up to 72 hours from dosing and by a 14-days washout period.
The blood sampling was performed through a peripheral venous catheter of adequate diameter and kept in a vein in one of the arms for multiple blood collections during hospitalization. The cannula was kept unobstructed by injecting 1 mL of a 100 UI/mL heparin solution in saline. One milliliter (1 mL) was withdrawn and discarded before each blood collection that was performed in 7.5 mL tubes containing lithium heparin as anticoagulant.
Blood samples were obtained prior to dosing (baseline) and 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 8.0, 10.0, 12.0, 16.0, 24.0, 36.0, 48.0, and 72.0 h post-dose. After each collection, the samples were immediately centrifuged at 3500 rpm for 10 minutes at 4ºC to separate plasma. The plasma obtained was transferred to pre-labeled cryogenic tubes in two aliquots for analysis and backup respectively and stored at -20ºC until shipment to bioanalytical laboratory for analysis.
The validation of the bioanalytical method was performed by high performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) for the imatinib quantification of in human heparinized plasma, meeting all the criteria for acceptability of specificity/selectivity, linearity, precision, accuracy, carry-over, ion suppression, recovery, robustness and stability tests.
Linearity was determined to assess the ability of the method to adequately relate the analyte concentration to the instrumental response. The straight-line equation was obtained and applied to the peak response (area ratios of plasma analyte vs internal standard): y = a + bx [weighted 1/x], where “y” is the response and “x” is the analyte concentration. Linearity was encountered for concentrations from 5.00 to 1,200.00 ng/mL.
The calculation of the samples concentration was based on the construction of a calibration curve for analyte with version 1.5 of the Analyst software data system. The function applied to the different samples of the calibration curve was calculated by a weighted linear regression system utilizing the relationship between the area of the analyte and the internal standard area (response) of the respective chromatograms and this function was validated according to the current legislation prior to evaluation of other parameters.
Within- and between-run precision were determined as the coefficient of variation, (CV) (%) = 100 (SD/M) and the accuracy (%) by 100 (M/T), where M is the mean, SD is the standard deviation of M, and T is the theoretical concentration. The Lower Limit of Quantification (LOQ) established by the method was 5.00 ng/mL, and the Quality Control (QC) samples validated were 15.00, 480.00 and 960.00 ng/mL. Additionally, the dilution process was validated with a 3,840.00 ng/mL QC sample.
Stability analysis was carried out in plasma (15, 480 and 960 ng/mL), subjected to three freeze-and-thaw cycles, short-term (6 h) at room temperature, as well as 69 h autosampler (6 ± 2 ºC) and long-term (164 days at -20oC).
Chemicals included ultrapure water from Millipore Purification System (Brazil), Methanol Liquid Chromatography grade from J.T. Baker (Mexico) and Ammonium Hydroxide pro-analysis grade from Carlo Erba (Italy). The reference materials used, imatinib and nilotinib (internal standard), were purchased from AK Scientific, Inc. (USA).
Standard stock solutions were prepared in pure methanol and working solutions in methanol/water (8/2; v/v). The mobile phase consisted of methanol/water (85/15; v/v) containing 10 mM of ammonia to elute isocratically the compounds.
A 25µL of each plasma sample was added into an Eppendorf tube followed by 25µL of internal standard (nilotinib 100 ng/mL) and 500 µL of methanol. The Eppendorf tubes were vortex-mixed for 30 seconds and centrifuged for 3 minutes at relative centrifuge force of 20800. The organic phase was transferred to a plastic tube and 4.5mL of methanol/water (80/20, v/v) was added before vortex-mix for 30 seconds. Finally, a 600 µL aliquot was transferred to an auto-injector (CTC Analytics, Switzerland) PCR 96-well polypropylene plate.
HPLC separation was performed using a Grace, Alltima HP, CI8 HL, 3um (100 x 4,6 mm i.d.) column and a Phenomenex, Gemini, C18, 5µm (4 x 3,0 mm i.d.) pre-column. The isocratic elution was performed using a binary pump (Agilent Technologies – Germany), and the mobile phase was composed of methanol/water (85/15; v/v) with 10mM of ammonia. The column temperature was maintained at 40oC and the pressure was typically 175 bar. The injection volume was 10 µL. The typical retention time obtained was 1.47 min and 1.97 min for imatinib and nilotinib respectively, and run time of 2.5 min.
The compounds were extracted from plasma samples and quantified by HPLC-MS/MS using an API5500Qtrap (Sciex/Applied Biosystems, Canada), equipped with positive electrospray ionization ion source, and detecting the analyte and internal standard (IS) using multiple reactions monitoring (MRM) with the transitions of m/z 494.3>217.2, and 530.2>289.2 respectively.
The parameters of the bioanalytical method used are summarized in Table 1.
Table 1. Summary of the bioanalytical method
|
Analyte |
Imatinib |
|
Internal standard |
Nilotinib |
|
Biological matrix |
human plasma |
|
Anticoagulant |
heparin |
|
Linearity |
5.00 to 1,200.00 ng/mL |
|
Curve equation |
y = a + bx (1/x) |
|
Lower Limit of Quantification (LIQ) |
5.00 ng/mL |
|
Low Quality Control (CQB) |
15.00 ng/mL |
|
Medium Quality Control (CQM) |
480.00 ng/mL |
|
High Quality Control (CQA) |
960.00 ng/mL |
|
Quantification parameter |
Response (analyte area/PI area) |
|
Detection parameter |
Signal-noise greater than 5 |
|
Post-processing stability time |
69 hours |
|
Freeze/thaw cycles |
3 cycles |
|
Short term stability time |
6 hours |
|
Long term stability time |
164 days |
To prove the bioequivalence between both formulations, and consequently their interchangeability, the analysis of variance (ANOVA) was performed on the ln-transformed data after normality testing. Based on the ANOVA results, a 90% confidence interval (CI) for the μT/μR (ratio of geometric means for the test and the reference product) of the analyzed pharmacokinetic parameters was constructed and the statistical significance of effects was determined on basis of the p values calculated, with significance values bigger than 0.05 which means no statistical significance. Bioequivalence was assumed when 90% CI of the point estimate for AUC0-t, AUC0-∞ and for Cmax.
The Adverse Events (AE) were classified following the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (in force at the time of the study, –2010) and recorded in the Case Report Forms (CRF).
RESULTS
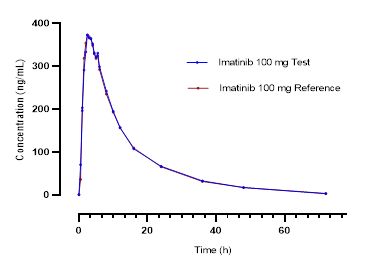
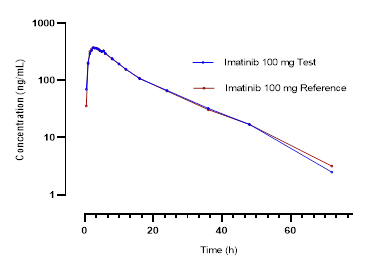
The statistical analysis of pharmacokinetic parameters described in the section Methods indicated that the geometric mean ratio of test to reference Cmax was 102.26%, with a 90% confidence interval (94.17-111.04%) and AUC0-t was 101.24%, with a 90% confidence interval (95.19-107.68%).
Figure 1 shows the mean curves of plasma concentrations of imatinib (reference and test drugs) versus time for the 42 volunteers.
|
a)
b)
|
|
Figure 1. Mean plasma concentrations of imatinib versus time for each formulation: (a) linear scale, (b) base 10 logarithmic scale |
Table 2 shows the mean pharmacokinetic parameters, standard deviation and coefficient of variation obtained from the plasma analysis of 42 volunteers submitted to the statistical analysis of the study between Glivec® (reference) and imatinib mesylate (test).
Table 2. Mean pharmacokinetic parameters
|
Pharmacokinetic parameters |
Glivec® |
Imatinib mesylate |
|
|
t1/2 (h) |
Arithmetic Mean |
12.57 |
12.19 |
|
Geometric Mean |
12.33 |
12.01 |
|
|
Minimum |
8.35 |
7.43 |
|
|
Maximum |
18.44 |
17.64 |
|
|
SD |
2.49 |
2.10 |
|
|
CV (%) |
19.80 |
17.25 |
|
|
tmax (h) |
Arithmetic Mean |
2.92 |
2.86 |
|
Geometric Mean |
2.55 |
2.59 |
|
|
Minimum |
1.00 |
1.00 |
|
|
Maximum |
8.00 |
5.50 |
|
|
SD |
1.60 |
1.25 |
|
|
CV (%) |
54.86 |
43.78 |
|
|
Cmax |
Arithmetic Mean |
451.75 |
452.10 |
|
Geometric Mean |
418.44 |
427.89 |
|
|
Minimum |
156.27 |
246.01 |
|
|
Maximum |
850.17 |
865.43 |
|
|
SD |
176.80 |
155.58 |
|
|
CV (%) |
39.14 |
34.41 |
|
|
AUC0-t |
Arithmetic Mean |
5265.10 |
5275.92 |
|
Geometric Mean |
4947.70 |
5009.08 |
|
|
Minimum |
1566.55 |
2054.98 |
|
|
Maximum |
9618.74 |
9661.52 |
|
|
SD |
1837.72 |
1727.50 |
|
|
CV (%) |
34.90 |
32.74 |
|
|
AUC0-∞ |
Arithmetic Mean |
5473.16 |
5496.82 |
|
Geometric Mean |
5161.00 |
5238.79 |
|
|
Minimum |
1629.06 |
2122.72 |
|
|
Maximum |
9732.13 |
9847.36 |
|
|
SD |
1841.57 |
1719.89 |
|
|
CV (%) |
33.65 |
31.29 |
|
|
AUC0-t /AUC0-∞ |
Arithmetic Mean |
0.96 |
0.96 |
|
Geometric Mean |
0.96 |
0.96 |
|
|
Minimum |
0.92 |
0.87 |
|
|
Maximum |
0.99 |
0.99 |
|
|
SD |
0.02 |
0.02 |
|
|
CV (%) |
2.13 |
2.46 |
|
Captions: SD = standard deviation; CV = coefficient of variation (%).
Formulations were well tolerated. After medical examination, the volunteers presented no clinically relevant alterations. No significant changes in the laboratory exams (hemogram, hepatic enzymes, renal function) and in the electrocardiogram post-study were observed.
During the study, the volunteers developed 123 AE (49.84% from test administration, 50.16% from reference) being classified as follows: 16 (13%) as possibly related to the study medication (seven headaches, seven changes in laboratory tests, one diarrhea and one dizziness, two (1.6%) unlikely related to the study medication (two headaches), and 105 (85.4%) unrelated to the study medication. Of these events, only two were considered severe, but not drug-related (polytrauma) and four were moderate (grade 2), all other events were mild (grade 1). Thirty (23%) of all AE required some type of management as medication or observation in the cases of diarrhea, headache, systemic arterial hypertension (one event) and dizziness.
DISCUSSION
This study was planned and performed in order to obtain the pharmacokinetic parameters Cmax, AUC0-t, and AUC0-∞. The values of the confidence intervals of each of these parameters were within the acceptable limit for the ratio between geometric averages of the test and reference products according to the current legislation.
The study design was drawn to assess comparative bioavailability between two formulations of imatinib mesylate coated tablet containing 100 mg using chromatography-mass spectrometry technique with optimized analytical run of 2.5 min. Several articles have been previously published presenting the analytical methodology with HPLC-UV and HPLC-MS22,25, i.e., respectively HPLC-ultraviolet and HPLC-mass spectrometry.
Headache was the adverse event with the highest incidence, similar to other studies11,26 earlier published and different from a study with a dose of 400mg, where there was a higher incidence of nausea, both in healthy volunteers and in patients with CML22. The incidence was 9 out of 16 events, where 7 were reported as possibly related to study drug, consistent with those described in the literature10,17,18,22. All AE were resolved during the study period.
The maximum concentration obtained in several publications seems to suggest a good correlation with the administered dose10,11. This indicates that therapeutic concentrations ranging between 1,000 and 1,400 ng/mL27 can be reached according to the guidelines in the Glivec® package insert, with both drugs utilized in the current investigation.
The high cost of cancer drugs, especially in developing countries, makes it difficult for patients to access the best available treatment. With new options for this product, it is expected that prices will fall back to more accessible levels which may eventually improve the patients’ quality of health.
Studies have shown that the inclusion of generic drugs in the treatment of CML improves patient adherence28. At the same time, in countries with centralized health systems, as in Brazil (SUS), the continuous government supply is critical, depending on long-term negotiation with pharmaceutical companies at lower costs than branded products and the competition from multiple market players (industry, distribution).
The results show that the test and reference formulations are bioequivalent and meet the criteria set forth in topic 3.2 f of Anvisa’s Resolution RE number 1170, dated April 19, 2006, in effect during the study, which states that two drugs are considered bioequivalent if the extreme values of the 90% confidence interval of the ratio of the geometric means (AUC0-t test / AUC0-t reference and Cmax test/ Cmax reference) are between 80% and 125%29.
CONCLUSION
Based on statistical results, both imatinib mesylate 100 mg coated tablet from Eurofarma Laboratórios S.A. and Glivec® 100 mg manufactured by Novartis Biociências S.A. (reference product) comply with the regulatory requirements to be considered bioequivalent. The test product is interchangeable with the reference based on their biopharmaceutical performance.
Both products were well tolerated by the participants in terms of AE, clinical examination, electrocardiogram, and laboratory assays. The adverse event profile concurs with the literature for the reference product and package insert.
Finally, the clinical use of generic drugs should be promoted as a cost-effective alternative for public health systems. Bioequivalence trials have demonstrated interchangeability between generic and reference drugs, ensuring the quality of treatment remains consistent.
CONTRIBUTIONS
All authors contributed equally to the wording and review of the manuscript and approved the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
FUNDING
This study was funded by Eurofarma Laboratórios. Itapevi, São Paulo, Brazil.
REFERENCES
1. An X, Tiwari AK, Sun Y, et al. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res. 2010;34(10):1255-68.
2. Kantarjian H, O'Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981-7.
3. Kantarjian HM, Smith TL, O'Brien S, et al. Prolonged survival in chronic myelogenous leukemia after cytogenetic response to interferon-alpha therapy. The Leukemia Service. Ann Intern Med. 1995;122(4):254-61.
4. Mughal TI, Radich JP, Deininger MW, et al. Chronic myeloid leukemia: reminiscences and dreams. Haematologica. 2016;101(5):541-58.
5. Sasaki K, Strom SS, O'Brien S, et al. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. The Lancet Haematology. 2015;2(5):e186-93.
6. Attwood MM, Fabbro D, Sokolov AV, et al. Trends in kinase drug discovery: targets, indications and inhibitor design. Nat Rev Drug Discov. 2021;20(11):839-61.
7. Mohajeri E, Kalantari-Khandani B, Pardakhty A, et al. Comparative pharmacokinetic evaluation and bioequivalence study of three different formulations of Imatinib Mesylate in CML patients. Int j hematol. 2015;9:165-72.
8. Santos SL, Morrone FB. Resultados do mesilato de imatinibe no tratamento da leucemia mielóide crônica: uma revisão bibliográfica. Rev Grad. [Internet]. 2008[acesso 2022 fev 17];1(1)1-14. Disponível em: https://revistaseletronicas.pucrs.br/ojs/index.php/graduacao/article/view/2845.
9. Al-Hadiya BM, Bakheit AH, Abd-Elgalil AA. Imatinib mesylate. Profiles Drug Subst Excip Relat Methodol. 2014;39:265-97.
10. Ostrowicz A, Mikołajczak PL, Wierzbicka M, et al. Bioequivalence study of 400 and 100 mg imatinib film-coated tablets in healthy volunteers. Acta Pol Pharm. 2014;71(5):843-54.
11. Jung JA, Kim N, Yang JS, et al. Bioequivalence study of two imatinib formulations after single-dose administration in healthy Korean male volunteers. Drug Res (Stuttg). 2014;64(12):651-5.
12. Kim KA, Park SJ, Kim C, et al. Single-dose, randomized crossover comparisons of different-strength imatinib mesylate formulations in healthy Korean male subjects. Clin Ther. 2013;35(10):1595-602.
13. Jawhari D, AlSwisi M. Bioavailability of a new generic formulation of imatinib mesylate 400mg tablets versus glivec in healthy male adult volunteers. J Bioequiv Availab. 2011;3(7):161-4.
14. Miura M. Therapeutic drug monitoring of imatinib, nilotinib, and dasatinib for patients with chronic myeloid leukemia. Biol Pharm Bull. 2015;38(5):645-54.
15. Rezende VM, Rivellis AJ, Gomes MM, et al. Determination of serum levels of imatinib mesylate in patients with chronic myeloid leukemia: validation and application of a new analytical method to monitor treatment compliance. Rev Bras Hematol Hemoter. 2013;35(2):103-8.
16. Qian Y, Sun L-N, Liu Y-J, et al. Genetic Polymorphisms and Adverse Events on Unbound Imatinib and Its Active Metabolite Concentration in Patients With Gastrointestinal Stromal Tumors. Front pharmacol. 2019;10:854. doi: https://doi.org/10.3389/fphar.2019.00854
17. Nikolova Z, Peng B, Hubert M, et al. Bioequivalence, safety, and tolerability of imatinib tablets compared with capsules. Cancer Chemother Pharmacol. 2004;53(5):433-8.
18. Ostrowicz A, Miko£ajczak PA, Wierzbicka M, et al. Bioequivalence study of 400 and 100 mg imatinib film-coated tablets in healthy volunteers. Acta Poloniae Pharmaceutica. 2014;71:843-54.
19. Pena MA, Muriel J, Saiz-Rodriguez M, et al. Effect of Cytochrome P450 and abcb1 polymorphisms on imatinib pharmacokinetics after single-dose administration to healthy subjects. Clin Drug Investig. 2020;40(7):617-28.
20. Suttorp M, Bornhauser M, Metzler M, et al. Pharmacology and pharmacokinetics of imatinib in pediatric patients. Expert Rev Clin Pharmacol. 2018;11(3):219-31.
21. Abou Dalle I, Kantarjian H, Burger J, et al. Efficacy and safety of generic imatinib after switching from original imatinib in patients treated for chronic myeloid leukemia in the United States. Cancer Med. 2019;8(15):6559-65.
22. Vargas M, Villarraga E. Bioequivalence study of imatinib formulations that contain 400 mg in healthy colombians. J Bioequiv Availab. 2017;9(5):483-8.
23. Glivec(R)® (mesilato de imatinibe) [bula na Internet]. São Paulo: Novartis Biociencias S.A; 2001. Bula de Remédio. [acesso 2022 fev 15]. Disponível em: https://consultas.anvisa.gov.br/#/bulario/q/?nomeProduto=GLIVEC
24. Conselho Nacional de Saúde (BR). Resolução n° 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília, DF. 2013 jun 13; Seção I:59.
25. Rezende VM, Rivellis A, Novaes MM, et al. Quantification of imatinib in human serum: validation of a high-performance liquid chromatography-mass spectrometry method for therapeutic drug monitoring and pharmacokinetic assays. Drug Des Devel Ther. 2013;7:699-710.
26. O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994-1004.
27. Garcia-Ferrer M, Wojnicz A, Mejia G, et al. Utility of therapeutic drug monitoring of imatinib, nilotinib, and dasatinib in chronic myeloid leukemia: a systematic review and meta-analysis. Clin Ther. 2019;41(12):2558-70.
28. Cole AL, Jazowski SA, Dusetzina SB. Initiation of generic imatinib may improve medication adherence for patients with chronic myeloid leukemia. Pharmacoepidemiol Drug Saf. 2019;28(11):1529-33.
29. Agência Nacional de Vigilância Sanitária (BR). Resolução RE 1.170 de 19 de Abril de 2006. Diário Oficial da União, Brasília, DF, 2006 abr 24. [acesso 2023 dez 7]. Disponível em: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2006/res1170_19_04_2006.html
Recebido em 10/7/2023
Aprovado em 29/11/2023
Associate-Editor: Mario Jorge Sobreira da Silva. Orcid iD: https://orcid.org/0000-0002-0477-8595
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde