ORIGINAL ARTICLE
Trk Inhibition Reduces Tumorsphere Formation and Changes Expression of Stemness Markers in SK-ES-1 Ewing Sarcoma Cells
Inibição de Trk Reduz a Formação de Tumoresferas e Modifica a Expressão de Marcadores Tronco Tumorais em Células de Sarcoma de Ewing SK-ES-1
Inhibición de Trk Reduce la Formación de Esferoides Tumorales y Cambia la Expresión de Marcadores de Stemness en Células de Sarcoma de Ewing SK-ES-1
https://doi.org/10.32635/2176-9745.RBC.2023v69n4.4262
Rafael Pereira dos Santos1; Bruna Almeida dos Santos2; Lauro José Gregianin3; André Tessainer Brunetto4; Algemir Lunardi Brunetto5; Rafael Roesler6; Caroline Brunetto de Farias7
1,2,6,7Universidade Federal do Rio Grande do Sul (UFRGS), Hospital de Clínicas de Porto Alegre (HCPA), Centro de Pesquisa Experimental, Laboratório de Câncer e Neurobiologia; Instituto de Ciências Básicas da Saúde, Departamento de Farmacologia. Porto Alegre (RS), Brazil. E-mails: rafa.rpds@gmail.com; balmeidast@gmail.com; rafaelroesler@hcpa.edu.br; carolbfarias@gmail.com. Orcid iD: https://orcid.org/0000-0003-0949-9648; Orcid iD: https://orcid.org/0000-0003-3256-5500; Orcid iD: https://orcid.org/0000-0001-6016-2261; Orcid iD: https://orcid.org/0000-0002-6435-6626
3UFRGS, HCPA, Escola de Medicina, Departamento de Pediatria; Serviço de Oncologia Pediátrica. Porto Alegre (RS), Brazil. E-mail: lgregianin@hcpa.edu.br. Orcid iD: https://orcid.org/0000-0003-0788-7858
4,5Instituto do Câncer Infantil. Porto Alegre (RS), Brazil. E-mails: andrebrunetto@ici.ong; institucional@ici.ong. Orcid iD: https://orcid.org/0000-0002-7958-1279; Orcid iD: https://orcid.org/0000-0003-0668-6894
Corresponding author: Caroline Brunetto de Farias. Rua São Manoel, 850 – Rio Branco. Porto Alegre (RS), Brazil. CEP 90620-110. E-mail: carolbfarias@gmail.com
ABSTRACT
Introduction: Ewing sarcoma (ES) is a highly aggressive type of childhood cancer characterized by a chromosomal translocation resulting in fusions between the gene encoding EWS RNA Binding Protein 1 (EWSR1) and one gene of the ETS family, most frequently FLI-1, resulting in the EWS-FLI1 aberrant transcription factor. ES tumors can contain a subpopulation of cells showing cancer stem cell (CSC) features, which express stemness markers including CD133, OCT4 (Octamer-binding transcription factor 4), and NANOG, and display capacity to form tumorspheres likely enriched in CSCs. Neurotrophin (NT) receptors of the tropomyosin receptor kinase (Trk) family (TrkA, TrkB, and TrkC) may play a role in stimulating ES progression, but their possible role in CSCs remains unknown. Objective: To verify the effect of Trks inhibition on the formation of tumorspheres as well as the gene expression of stem markers. Method: The cells were dissociated and the formation of spheres was induced with supplemented culture medium and the K252a treatment was performed. After RNA extraction, mRNA expression levels of target genes Prom1 (CD133), OCT4 (POU5F1), SOX2, and Musashi-1 (MSI1) were analyzed by qPCR. Results: The pan-Trk inhibitor K252a (100 or 500 mM) hindered tumorsphere formation in human SK-ES-1 ES cell cultures. K252a also reduced mRNA expression of Prom1 (CD133-coding gene) while enhancing expression of OCT4. No changes in mRNA levels of SOX2 or Musashi-1 were observed. Conclusion: These findings provide the first evidence suggesting that Trk activity can influence stemness in ES cells.
Key words: Receptor, trkA; receptors, nerve growth factor; neoplastic stem cells; sarcoma, Ewing.
RESUMO
Introdução: O sarcoma de Ewing (SE) é um tipo altamente agressivo de câncer infantil caracterizado por uma translocação cromossômica que resulta em fusões entre o gene que codifica a proteína de ligação a RNA EWS 1 (EWSR1) e um gene da família ETS, mais frequentemente o FLI-1, resultando no fator de transcrição aberrante EWS-FLI1. Os tumores de SE podem conter uma subpopulação de células com características de células-tronco tumorais (CTT), que expressam marcadores de pluripotência como CD133, OCT4 e NANOG, e têm a capacidade de formar esferas tumorais provavelmente enriquecidas em CTT. Os receptores de neurotrofinas (NT) da família de receptor de quinase de tropomiosina (Trk) (TrkA, TrkB e TrkC) podem desempenhar um papel no estímulo à progressão do SE, mas seu possível papel nas CTT permanece desconhecido. Objetivo: Verificar o efeito da inibição dos Trk na formação de tumoresferas, bem como na expressão gênica de marcadores de pluripotência. Método: As células foram dissociadas, a formação de esferas com meio de cultura suplementado foi induzida e realizou-se o tratamento com K252a. Após a extração de RNA, os níveis de expressão de mRNA dos genes-alvo Prom1 (CD133), OCT4 (POU5F1), SOX2 e Musashi-1 (MSI1) foram analisados por qPCR. Resultados: O inibidor pan-Trk K252a (100 ou 500 mM) impediu a formação de esferas tumorais em culturas de células de SE humanas SK-ES-1. O K252a também reduziu a expressão de mRNA de Prom1 (o gene que codifica CD133), enquanto aumentou a expressão de OCT4. Não foram observadas mudanças nos níveis de mRNA de SOX2 ou Musashi-1. Conclusão: Essas descobertas fornecem as primeiras evidências, sugerindo que a atividade dos Trk possa influenciar a pluripotência nas células de SE.
Palavras-chave: receptor trkA; receptores de fator de crescimento neural; células-tronco neoplásicas; sarcoma de Ewing.
RESUMEN
Introducción: El sarcoma de Ewing (SE) es un tipo de cáncer infantil altamente agresivo caracterizado por una translocación cromosómica que resulta en fusiones entre el gen que codifica la proteína de unión a RNA EWS 1 (EWSR1) y un gen de la familia ETS, más frecuentemente FLI-1, lo que resulta en el factor de transcripción aberrante EWS-FLI1. Los tumores del SE pueden contener una subpoblación de células que presentan características de células madre cancerosas (CMC), las cuales expresan marcadores de pluripotencia como CD133, OCT4 y NANOG, y muestran la capacidad de formar esferas tumorales probablemente enriquecidas en CMC. Los receptores de neurotrofinas (NT) de la familia del receptor de quinasa de tropomiosina (Trk) (TrkA, TrkB y TrkC) podrían desempeñar un papel en el estímulo de la progresión del SE, pero su posible papel en las CMC aún es desconocido. Objetivo: Verificar el efecto de la inhibición de los Trk en la formación de esferoides tumorales, así como en la expresión génica de marcadores de pluripotencia. Método: Las células fueron disociadas e inducidas a formar esferas con un medio de cultivo suplementado y se realizó el tratamiento con K252a. Después de la extracción de ARN, los niveles de expresión de ARNm de los genes objetivo Prom1 (CD133), OCT4 (POU5F1), SOX2 y Musashi-1 (MSI1) se analizaron mediante qPCR. Resultados: El inhibidor pan-Trk K252a (100 o 500 mM) evitó la formación de esferas tumorales en cultivos de células de SE humanas SK-ES-1. El K252a también redujo la expresión de ARNm de Prom1 (el gen que codifica CD133), mientras que aumentaba la expresión de OCT4. No se observaron cambios en los niveles de ARNm de SOX2 o Musashi-1. Conclusión: Estos hallazgos proporcionan las primeras evidencias que sugieren que la actividad de Trk puede influir en la pluripotencia en las células del SE.
Palabras clave: receptor trkA; receptores de factor de crecimiento nervioso; células madre neoplásicas; sarcoma de Ewing.
INTRODUCTION
A chromosomal translocation resulting in fusions between the gene encoding EWS RNA Binding Protein 1 (EWSR1) and one gene of the ETS family, most frequently FLI-1, is the genetic hallmark of Ewing sarcoma (ES), a highly aggressive cancer type that afflicts mostly children and adolescents. The resulting EWS-FLI1 protein acts as an aberrant transcription factor that reprograms many aspects of gene expression and epigenetic regulation1,2. Despite the increased survival in patients with localized disease, reaching a 5-year survival rate of up to 70% in the best centers3,4, unfortunately, nearly 20% of patients progress to recurrent or refractory disease. Additionally, one-third of patients already present with metastatic disease at the time of diagnosis, with these features being the most critical in terms of prognosis. Survival in this scenario varies between 10% and 20%1. ES tumors contain between 3% to 15% of cells showing cancer stem cell (CSC) features. ES CSCs are characterized by expressing stemness markers CD133, OCT4 and NANOG, forming tumorspheres under low attachment conditions in serum-free medium, and initiating tumors when xenografted into nonobese diabetic (NOD)/severe combined immunodeficiency (SCID) mice5-7. CSCs have the ability for self-renewal and unrestricted differentiation. The presence and activity of multiple transporters make CSCs a population of tumor cells constitutively resistant to therapy. There are also other mechanisms of resistance of CSCs to treatment, such as the activity of mediators of epithelial-mesenchymal transition and crosstalk with non-tumor cells within the tumor microenvironment that favor the survival and expansion of CSCs8.
Neurotrophins (NTs) are growth factors that regulate neuronal development, survival and plasticity9,10. NT actions are mediated by activation of cell surface receptors belonging to the tropomyosin receptor kinase (Trk) family. Trk receptors TrkA, TrkB, and TrkC are encoded by the NTRK1, NTRK2, and NTRK3 genes respectively and activated by nerve growth factor (NGF, which binds TrkA), brain-derived neurotrophic factor (BDNF, which is the primary ligand for TrkB), NT-3, which binds all three Trk types, and NT-4/5, which binds TrkB. Activation of Trks leads to receptor homodimerization and phosphorylation of tyrosine residues, which in turn stimulate intracellular cell signaling mediated by multiple protein kinase pathways11,12. Although Trks are being increasingly investigated as anticancer targets13,14 and may be involved in ES growth15, their possible role in ES CSCs remains unknown.
The role of neurotrophin inhibition in ES has been demonstrated; however, its mechanism still remains poorly understood. A greater understanding of these mechanisms, their impact at the level of tumor stem cells, and their effects on signaling pathways is necessary to enhance the possibility of proposing a treatment alternative. This involves subverting resistance processes and minimizing the toxicity of conventional therapies. Therefore, the objective of this article work is to show the relation of Trk and tumorsphere formation and the effects of its inhibition on mRNA expression of stemness genes in human SK-ES-1 ES cells.
METHOD
Human SK-ES-1 cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA) and checked for authenticity and lack of contamination. Cells were grown in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA), containing 0.1% Fungizone (250 mg/kg; Invitrogen, São Paulo, Brazil), 100 U/l gentamicin (4 mg/ml; Nova Pharma, Jardim Anápolis, Brazil), 50 mg/ml ampicillin (Nova Pharma), and 10% fetal bovine serum (FBS, Invitrogen, São Paulo, Brazil), at 37 °C in a humidified incubator under 5% CO2.
Cells were dissociated with trypsin-EDTA into single cell suspension and seeded at 2 × 103 cells/well in 24-well plates. The cells were cultured in a serum-free tumorsphere (TS)-inducing medium, containing DMEM-F12 (1:1) supplemented with 2% B27 supplement (Gibco, Invitrogen, Walthan, USA), 20 ng/ml recombinant human EGF (Sigma-Aldrich, MO, USA), 20 ng/ml human leukemia inhibitor factor (Thermo Fisher Scientific, Life Technologies, Walthan, USA), 10 IU/ml (5 mg/ml) heparin (Roche, Mannheim, Germany) and antibiotics. Media was changed every 4 days. After 8-9 days, tumorspheres were dissociated with a non-enzymatic solution containing 1 mM EDTA, 40 mM Tris-HCl and 150 mM NaCl, and re-plated in a 96-well plate to evaluate their capacity to self-renew through secondary ES tumorsphere formation. Tumorspheres with at least 80 μm were analyzed and quantified by inverted phase microscopy (Leica Microsystems, Mannheim, Germany). The effect of K252a 100 nM in tumorsphere formation capacity across 3 days was examined as previously described16.
Tumorspheres were dissociated and total RNA was extracted from ES cells using TRIzol (Invitrogen). Prior to RT-PCR, the samples were treated with DNase I (Promega Corporation, Madison, USA). Total RNA was quantified with NanoDrop (Thermo Fisher Scientific). SuperScriptTM First-Strand Synthesis System for RT-PCR (Invitrogen) enabled first strand synthesis. Messenger RNA (mRNA) expression levels of target genes Prom1 (CD133), OCT4 (POU5F1), SOX2, Musashi-1 (MSI1) and housekeeping gene β-Actin were quantified using KiCqStartTM SYBR Green qPCR ReadyMixTM (Sigma-Aldrich, St. Louis, USA) in triplicate reactions. The gene expression levels were calculated using the ΔCT. Cycling parameters were as follows: 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 3 s, annealing at 60°C for 15s, and extension at 72°C for 30 s. Untreated cells were used as calibrator.
All data are shown as CT means ± standard deviation (SD) of three independent experiments. Differences between mean values were evaluated by one-way analysis of variance (ANOVA) followed by the Tukey or Bonferroni multiple comparisons tests in GraphPad Prism 9.0. In all comparisons, p < 0.05 was considered to indicate statistically significant differences.
In compliance with Directive 510/201617 of the National Health Council, the Institutional Review Board waived the analysis and approval of the present study.
|
Table 1. Primer sequences used for Real-Time qPCR measurement of mRNA levels of stem cell markers genes in cells from human SK-ES-1 ES tumorspheres |
||||||||||||
Captions: F= forward; R = reverse. |
RESULTS
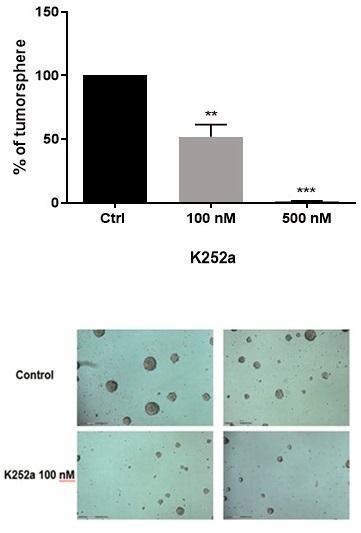
Treatment with the Trk inhibitor K252a at 100 and 500 nM for 72 h resulted in a dose-dependent impairment in tumorsphere formation. A significant decrease in tumorsphere formation expressed as tumorsphere number and size is apparent in drug-treated cell cultures, as follows (Figure 1 – K252a 100 nM, p = 0.0063; K252a 500 nM, p = 0.0007).
|
|
|
Figure 1. Analysis of tumorsphere formation in human SK-ES-1 ES cell cultures by number accounting and size. As shown, both drug doses reduced the percentage of ES cells aggregated as tumorspheres. A. Plotting of tumorsphere number percentage expressed as mean ± SD showing percentage of decrease in tumorsphere number when cells were treated with the 100 and 500 nM of K252a drug in different concentrations compared to control cells (n = three independent experiments). ** p = 0.0063 and ***p = 0.0007. B. Photomicrographies of cells from cultures of control (upper panels) and 100 nmM K252a treated (lower panels). Magnification: 10X |
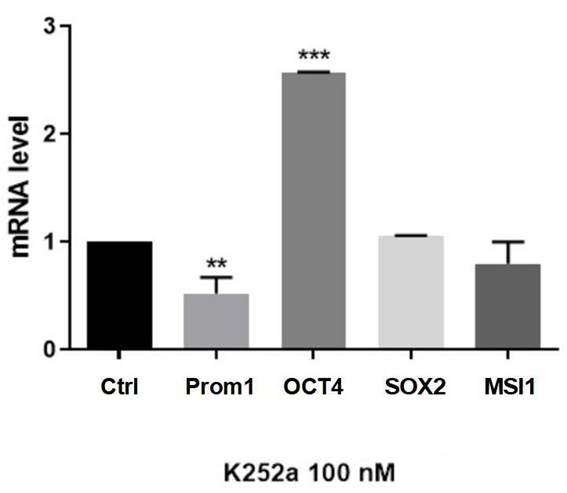
Primers used for quantitative RT-PCR measurement of mRNA levels in SK-ES-1 ES cells are shown in Table 1 (Method). A significant decrease in Prom1 (CD133) was observed upon treatment with K252a (100 nM; p = 0.0063 compared to controls), whereas increased expression was observed for OCT4 (p = 0.0001 compared to controls). No changes in mRNA levels of SOX2 and Musashi-1 were found (Figure 2).
|
|
|
Figure 2. Trk inhibition induces diverse responses in mRNA expression of stem cell genes. Tumorsphere mRNA levels were measured by Real-Time qPCR after three days of 100 nM K252a treatment. β-actin were used as endogenous gene and untreated cells was used as calibrator. Data are shown as mean ± SD of three independent experiments. K252a induced a significant decrease in Prom1 and an increase in OCT4 (POU5F1) expressions. No changes were observed for SOX2 and Musashi-1 (MSI1) expressions. **p = 0.0063 and ***p = 0.0001. |
DISCUSSION
Previously it has been shown that the pan-Trk inhibitor K252a impairs ES cell proliferation and re-sensitizes ES cells to cytotoxic chemotherapeutics18. However, previous studies have not examined a possible role for Trk in ES CSCs or whether Trk signaling can alter stemness in ES. Additionally it has been reported that the effectiveness of a drug can only become effective after its action against various mechanisms of tumor resistance, including CSCs. Therefore, the role of neurotrophin pathway inhibition was evaluated in ES CSCs, inducing the formation of tumorspheres from the SK-ES-1 lineage and treating these cells with K252a Trk inhibitor. Here, it was shown that Trk inhibition reduces tumorsphere formation capability in SK-ES-1 ES cells, along with a clear reduction in sphere size. This is the first study demonstrating the inhibition of enriched culture of ES CSC with a neurotrophin inhibitor. This result reaffirms the potential use of Trk inhibitors in therapy, as some drugs used in the clinic may have toxicity potential for adherent cells in culture but do not exhibit the same outcome in CSC culture, as is the case with doxorubicin19. With regard to the expression of stemness markers, expression of PROM1 was reduced and the expression of OCT4 was increased upon treatment with K252a.
CSC populations have been identified in ES, and stemness markers defining these subpopulations within Ewing´s sarcoma tumor have been proposed6,7,20. A subset of cells expressing CD133 isolated from ES tumors is capable of forming tumorspheres and initiating tumors in NOD/SCID mice. Results from experiments using serial transplantation of either CD133+ and CD133− cells derived from ES primary xenografts show that CD133+ cells but not CD133− cells can generate tumors in secondary and tertiary xenotransplantations. In addition to CD133, these ES cells show high expression of stem cell markers OCT4 and NANOG5. SOX2 is a stemness marker found in CSCs from ES tumors21,22 as well as ES CSC-like cells produced by EWS-FLI1 expression in hMSCs23.
The intracellular RNA-binding protein Musashi-1 is involved in stem cell renewal and pluripotency23, but it is not known whether it plays a role in ES. Transcription levels of MSI-1 were not altered during the inhibition of TRK with K252a, which can reinforce that this gene is not involved in ES stemness. EWS-FLI-1 fusion gene can induce expression of OCT4, SOX2, and NANOG in pediatric human mesenchymal stem cells (hMSCs). ES CSCs share a set of stemness genes with bone marrow-derived hMSCs4, and pediatric hMSCs harboring EWS-FLI-1 generate an ES stem cell-like subpopulation highly expressing Prom1, OCT4, and SOX2, among other markers, indicating that EWS-FLI-1 can act as an oncogenic event that reprograms ES cells to display a stem cell phenotype22.
There were not previous studies showing opposite effects of an experimental intervention on CD133 and OCT4 expression at either the mRNA or protein level. Although some studies demonstrate that the expression of CD133 is associated with metastasis and a worse prognosis, and that when CD133 is more expressed, OCT4 is also more expressed, however, the present findings show that after treatment with K252a there was decreased expression of CD133 and, in opposite way, increased OCT4 expression. This could potentially be a compensatory response of these cells, seeking to maintain their growth characteristics and treatment resistance, which was associated with reduced capacity to form tumorspheres. This is consistent with previous evidence that silencing OCT4 in breast cancer cells can induce epithelial-to-mesenchymal transition, which can directly promote stemness24, demonstrating that increased OCT4 expression could impair a stemness profile.
CONCLUSION
By evaluating tumorsphere formation capacity and stemness marker expression, the first evidence suggesting that pharmacological manipulation of Trk activity can affect stemness in ES was presented.
CONTRIBUTIONS
Rafael Pereira dos Santos, Bruna Almeida dos Santos contributed to the study design, experiments, data analysis, wording and review of the article. Lauro Gregianin contributed to the study design, data analysis and review. Algemir Lunardi Brunetto contributed to the study design and data analysis. André Tessainer Brunetto contributed to the study design, data analysis, wording and review. Rafael Roesler, Caroline Brunetto de Farias contributed to the study design and experiments, data analysis, and review. All the authors approved the final version to be published.
DECLARATION OF CONFLICT OF INTEREST
None.
FUNDING SOURCES
PRONON (Programa Nacional de Apoio à Atenção Oncologica)/Ministério da Saúde, Brasil (number 25000.202751/2016-65); Instituto do Câncer Infantil (ICI); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, number 305647/2019-9 R.R.); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Fundo de Incentivo à Pesquisa do Hospital de Clínicas (FIPE-HCPA).
REFERENCES
1. Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184-92. doi: https://doi/org/10.1016/S1470-2045(09)70286-4
2. Crompton BD, Stewart C, Taylor-Weiner A, et al. The genomic landscape of pediatric Ewing sarcoma. Cancer Discov 4. 2014;4(11):1326-4. doi: https://doi.org/10.1158/2159-8290.CD-13-1037
3. Ross KA, Smith NA, Murawski CD, et al. The biology of Ewing sarcoma. ISRN Oncol. 2013;2013;759725. doi: https://doi.org/10.1155/2013/759725
4. Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694-701. doi: https://doi.org/10.1056/nejmoa020890
5. Suvà ML, Riggi N, Stehle JC, et al. Identification of cancer stem cells in Ewing's sarcoma. Cancer Res 69(5):1776-81. https://doi.org/10.1158/0008-5472.can-08-2242
Hotfilder M, Mallela N, Seggewiß J, et al. Defining a characteristic gene expression set responsible for cancer stem cell-like features in a sub-population of Ewing sarcoma cells CADO-ES1. Int J Mol Sci. 2018;19:3908. https://doi.org/10.3390/ijms19123908
6. Santos RP, Gregianin L, Brunetto AT, et al. Cancer stem cells and chemoresistance in Ewing sarcoma. Curr Stem Cell Res Ther. 2023;18(7):926-36. doi: https://doi.org/10.2174/1574888x17666220627114710
Okugawa Y, Tanaka K, et al. Brain-derived neurotrophic factor/tropomyosin-related kinase B pathway in gastric cancer. Br J Cancer. 2013;108(1):121-30. doi: https://doi.org/10.1038/bjc.2012.499
7. Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14(23):2919-37. doi: https://doi.org/10.1101/gad.841400
8. Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14(1):7-23. doi: https://doi.org/10.1038/nrn3379
9. Schecterson LC, Bothwell M. Neurotrophin receptors: Old friends with new partners. Dev Neurobiol. 2010;70(5):332-8. doi: https://doi.org/10.1002/dneu.20767
10. Bothwell M. Recent advances in understanding neurotrophin signaling. F1000Res. 2016;28:5:F1000. doi: https://doi.org/10.12688/f1000research.8434.1
11. Thiele CJ, Li Z, McKee AE. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res 2009;15:5962-7. doi: https://doi/org/10.1158/1078-0432.CCR-08-0651
12. Roesler R, Farias CB, Abujamra AL, et al. BDNF/TrkB signaling as an anti-tumor target. Expert Rev Anticancer Ther. 2011;11:1473-5. doi: https://doi.org/10.1586/era.11.150
13. Heinen TE, Santos RP, Rocha A, et al. Trk inhibition reduces cell proliferation and potentiates the effects of chemotherapeutic agents in Ewing sarcoma. Oncotarget 2016;7(23):34860-80. doi: https://doi.org/10.18632/oncotarget.8992
14. Souza BK, Costa Lopez PL, Menegotto PR, et al. Targeting histone deacetylase activity to arrest cell growth and promote neural differentiation in Ewing sarcoma. Mol Neurobiol. 2018;55(9):7242-58. doi: https://doi.org/10.1007/s12035-018-0874-6
15. Conselho Nacional de Saúde (BR). Resolução n° 510, de 7 de abril de 2016. Dispõe sobre as normas aplicáveis a pesquisas em Ciências Humanas e Sociais cujos procedimentos metodológicos envolvam a utilização de dados diretamente obtidos com os participantes ou de informações identificáveis ou que possam acarretar riscos maiores do que os existentes na vida cotidiana, na forma definida nesta Resolução [Internet]. Diário Oficial da União, Brasília, DF. 2016 maio 24 [acesso 2023 dez. 28]; Seção I:44. Disponível em: http://bvsms.saude.gov.br/bvs/saudelegis/cns/2016/res0510_07_04_2016.html
16. Komuro H, Saihara R, Shinya M, et al. Identification of side population cells (stem-like cell population) in pediatric solid tumor cell lines. J Pediatr Surg. 2007;42(12):2040-5. doi: https://doi.org/10.1016/j.jpedsurg.2007.08.026
17. Cornaz-Buros S, Riggi N, Devito C, et al. Targeting cancer stem–like cells as an approach to defeating cellular heterogeneity in Ewing sarcoma. Cancer Res. 2014;74(22):6610-22. doi: https://doi.org/10.1158/0008-5472.CAN-14-1106
18. Fujii H, Honoki K, Tsujiuchi T, et al. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. 2009;34(5):1381-6.
19. Guzel Tanoglu E, Ozturk S. miR-145 suppresses epithelial-mesenchymal transition by targeting stem cells in Ewing sarcoma cells. Bratisl Lek Listy. 2021;122(1):71-7. doi: https://doi.org/10.4149/bll_2021_009
20. Riggi N, Suvà ML, De Vito C, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24(9):916-32. doi: https://doi.org/10.1101/gad.1899710
21. Forouzanfar M, Lachinani L, Dormiani K, et al. Intracellular functions of RNA-binding protein, Musashi1, in stem and cancer cells. Stem Cell Res Ther. 2020;11:193. doi: https://doi.org/10.1186/s13287-020-01703-w
22. Hu J, Qin K, Zhang Y, et al. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochem Biophys Res Commun. 2011;411:786-91. doi: https://doi/org/10.1016/j.bbrc.2011.07.025
Recebido em 25/8/2023
Aprovado em 27/12/2023
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde