ARTIGO ORIGINAL
Relation between Delay Time to Surgical Treatment of Prostate Cancer and Disease Recurrence Risk
Relação entre Tempo de Atraso no Tratamento Cirúrgico do Câncer de Próstata e Risco de Recorrência da Doença
Relación entre el Tiempo de Demora Hasta el Tratamiento Quirúrgico del Cáncer de Próstata y el Riesgo de Recurrencia de la Enfermedad
https://doi.org/10.32635/2176-9745.RBC.2024v70n1.4406
Denny Fabrício Magalhães Veloso1; Denise Sena Veloso2; André Felipe Zuccolo Barragat de Andrade3
1-3Universidade Federal de São João del-Rei. São João del-Rei (MG), Brasil.
1E-mail: silvaivg@gmail.com. Orcid iD: https://orcid.org/0000-0002-1131-6006
2E-mail: denisesv@ufsj.edu.br. Orcid iD: https://orcid.org/0000-0002-8066-5066
3E-mail: andrebarragat@ufsj.edu.br. Orcid iD: https://orcid.org/0000-0002-0041-7258
Corresponding author: Denny Fabrício Magalhães Veloso. Universidade Federal de São João del-Rei, Campus Centro-Oeste. Rua Sebastião Gonçalves Coelho, 400 – Chanadour. Divinópolis (MG), Brasil. CEP 35501-296. E-mail: silvaivg@gmail.com
ABSTRACT
Introduction: There is no consensus in the literature on a reasonable delay time from diagnosis to radical prostatectomy (RP) surgery, without worsening the prognosis. Objective: To evaluate the influence of the delay on the risk of disease recurrence in patients with acinar adenocarcinoma of the prostate treated with RP. Method: Four hundred and twelve patients undergoing RP were retrospectively evaluated. Of these, 172 were excluded due to incomplete data and another 28 due to preoperative staging as high-risk prostate cancer (PSA > 10 ng/mL or Gleason score on biopsy > 7). Pre- and postoperative stagings were compared and survival analysis was performed using the Kaplan-Meier method to investigate the influence of time on discordance between pre- and postoperative stagings. Results: For the 212 patients of the sample, the average time from diagnosis to RP was 176.1 ± 120.2 days (median 145.5 days), ranging from 29 to a maximum of 798 days. The Kaplan-Meier curve indicated that the cancer worsened the longer the delay between diagnosis and surgery. Patients undergoing surgery within 60 days had an approximately 95% probability of not increasing the initial risk of recurrence. This number fell to 80%, 70% and 50% in patients operated on up to 100, 120 and 180 days, respectively. Conclusion: Delay in performing RP represents a continuous risk of relapse. The ideal time for RP is up to 60 days from prostate biopsy, as the probability of upstaging is less than 5% in this period.
Key words: Prostatic Neoplasms; Prostatectomy; Time-to-Treatment; Disease Progression; Neoplasm Recurrence, Local.
RESUMO
Introdução: De acordo com a literatura, não há consenso sobre um tempo de atraso razoável desde o diagnóstico até a operação da prostatectomia radical (PR) sem piora do prognóstico. Objetivo: Avaliar a influência desse tempo no risco de recorrência da doença em pacientes com adenocarcinoma acinar da próstata tratados com PR. Método: Quatrocentos e doze pacientes submetidos à PR foram avaliados retrospectivamente. Destes, 172 foram excluídos por dados incompletos e outros 28, por estadiamento pré-operatório como câncer de próstata de alto risco (PSA > 10 ng/mL ou escore de Gleason na biópsia > 7). Os estadiamentos pré e pós-operatórios foram comparados, e a análise de sobrevida feita pelo método de Kaplan-Meier para examinar a influência do tempo na discordância entre os estadiamentos pré e pós-operatórios. Resultados: Para os 212 pacientes da amostra, o tempo médio desde o diagnóstico até a PR foi de 176,1 ± 120,2 dias (mediana de 145,5 dias), variando de 29 a um máximo de 798 dias. A curva de Kaplan-Meier indicou que o câncer piorava quanto maior o atraso entre o diagnóstico e a operação. Pacientes submetidos à cirurgia dentro de 60 dias tiveram cerca de 95% de probabilidade de não aumentarem o risco inicial de recorrência. Esse número caiu para 80%, 70% e 50% nos pacientes operados em até 100, 120 e 180 dias, respectivamente. Conclusão: O atraso na realização da PR representa risco contínuo de recorrência da neoplasia. O tempo ideal para PR é de até 60 dias a partir da biópsia da próstata, uma vez que a probabilidade de upstaging é inferior a 5% nesse período.
Palavras-chave: Neoplasias da Próstata; Prostatectomia; Tempo para o Tratamento; Progressão da Doença; Recidiva Local de Neoplasia.
RESUMEN
Introducción: Según la literatura, no existe consenso sobre un tiempo razonable de retraso desde el diagnóstico hasta la cirugía de prostatectomía radical (PR), sin empeorar el pronóstico. Objetivo: Evaluar la influencia de este tiempo sobre el riesgo de recurrencia de la enfermedad en pacientes con adenocarcinoma acinar de próstata tratados con PR. Método: Se evaluaron retrospectivamente 412 pacientes sometidos a PR. De ellos, 172 fueron excluidos por datos incompletos y otros 28 por estadificación preoperatoria como cáncer de próstata de alto riesgo (PSA > 10 ng/mL o puntuación de Gleason en la biopsia > 7). Se compararon las estadificaciones pre y posoperatorias y se realizó un análisis de supervivencia utilizando el método de Kaplan-Meier para examinar la influencia del tiempo en la discordancia entre las estadificaciones pre y posoperatorias. Resultados: Para los 212 pacientes de la muestra, el tiempo promedio desde el diagnóstico hasta la PR fue de 176,1 ± 120,2 días (mediana 145,5 días), oscilando entre 29 y 798 días. La curva de Kaplan-Meier indicó que el cáncer empeoraba cuanto mayor era el retraso entre el diagnóstico y la cirugía. Los pacientes sometidos a cirugía dentro de los 60 días tenían aproximadamente un 95% de probabilidad de no aumentar el riesgo inicial de recurrencia. Esta cifra cayó al 80%, 70% y 50% en los pacientes operados hasta 100, 120 y 180 días, respectivamente. Conclusión: El retraso en la realización de la PR representa un riesgo continuo de restablecimiento de la neoplasia. El momento ideal para la PR es hasta los 60 días desde la biopsia de próstata, ya que la probabilidad de upstaging es inferior al 5% en este periodo.
Palabras clave: Neoplasias de la Próstata; Prostatectomía; Tiempo de Tratamiento; Progresión de la Enfermedad; Recurrencia Local de Neoplasia.
INTRODUCTION
Prostate cancer is one of the most prevalent types of cancer in the world. In Brazil, the National Cancer Institute (INCA) estimates 66 thousand new occurrences each year until 20221. Radical prostatectomy (RP) is among its mainstay therapeutic options; therefore, during preoperative patient evaluation, it is important to determine clinical staging, which could diverge from postoperative pathological staging. The literature ascribes this divergence to a number of factors, among which, waiting time between diagnosis and surgical treatment2-6.
Disease progression occurs as a result of factors related to the individual and intrinsic to the histological tumor type as a result of tumor-host interaction7. Nam et al.8 demonstrated that a delay to surgery of six months or more is associated with greater risk of high-grade disease on analysis of the surgical specimen, and with early biochemical recurrence rates. Conversely, other studies suggest that time from diagnosis to surgery does not significantly impact disease progression2,9,10. These studies posit that the discrepancies between clinical and pathological staging are mostly related to errors in preoperative staging rather than to progression of the disease itself.
Although preoperative parameters such as biopsy Gleason score, digital-rectal examination, and PSA level are acknowledged prostate cancer prognostic predictors in men diagnosed with the disease, treatment delay remains a debated issue and is yet to be throroughly investigated9. Prior research has so far failed to confirm a reasonably safe time threshold for performing RP without worsening prognosis9. Determining such a time limit to operation could inform and underpin routine clinical practice and future public health policies aiming to provide prostate cancer patients with quality treatment11.
This paper aims to evaluate
the influence of time delay from prostate cancer diagnosis to RP operation on
disease recurrence risk in patients with acinar adenocarcinoma of the prostate
who underwent RP.
METHOD
This investigation was conducted with prior approval from the ethics committees of the institutions involved and in compliance with the recommendations of the Brazilian Ministry of Health and the Research Ethics guidelines of major international documents guiding research involving human subjects (Ministry of Health, Resolution 466/201212; CAAE (submission for ethical review): 12660619.7.0000.5130).
The records of 412 patients diagnosed with prostate cancer who underwent RP between January, 2013, and April, 2018, at two reference public hospitals in the state of Minas Gerais, Brazil were evaluated retrospectively. Data were collected from electronic medical records, one by one by the main researcher, no other data source was used. Patients whose clinical records lacked sufficient information for data collection or patients who previously underwent any kind of preoperative oncological treatment, such as hormone therapy, chemotherapy or radiotherapy, were excluded from the study.
Based on D’Amico et al.7’s proposed criteria, all patients were classified according to disease recurrence risk in both pre- and post-operative periods. D'Amico et al.7 proposed the stratification of risk groups into categories: Low risk (PSA<10 ng/mL, Gleason score ≤ 6 and stage T1-T2a), intermediate risk (PSA 10 to 20 ng/mL, Gleason score = 7 and stage T2b) and high risk (PSA>20 ng/mL, Gleason score ≥ 8 and stage T2c-T2a). Preoperative data analysis contemplated: PSA value at time of diagnosis, TNM classification determined by digital rectal exam, and biopsy Gleason score that confirmed prostatic neoplasia diagnosis. Postoperatively, TNM was established by pathological examination of the surgical specimen.
After determining recurrence risk classifications, patients clinically classified at high risk were excluded, given the impossibility of assessing the worsening risk of recurrence in these patients using the criteria of D’Amico et al.7. The preoperative classifications were then compared with the postoperative classifications for each patient. The time between diagnosis and surgical treatment was calculated in days by simple difference between the date of the ultrasound-guided biopsy and the date of the operation.
Survival analysis was done by Kaplan-Meier method to examine the influence of time on the discordance between pre- and post-operative staging. The response variable was time from initial observation to a subsequent event. In this case, the event was recurrence risk progression, determined as upstaging. Patients whose recurrence risks were concordant and those who for any reason presented recurrence risk reduction after RP were censored on the date of operation. A tool for survival rate analysis was utilized to estimate the probability of each subject’s initial recurrence risk not worsening in the time till RP. In this study, zero time was considered as date of prostate cancer diagnosis and event of interest as recurrence risk progression. Level of significance considered was p < 0.05.
RESULTS
Four hundred and twelve medical records were assessed, of which 172 were excluded because of incomplete data. Analysis of 240 patients indicated ages from 44 to 78 years, median of 64 years, and mean of 64 ± 6.9 years, distributed according to recurrence risk as shown in Table 1. Twenty-eight patients clinically classified as high-risk were excluded from the sample.
Table 1. Classification of recurrence risk in 240 patients with prostate cancer, before and after RP according to D’Amico et al.‘s proposed categories7
|
Before RP |
After RP |
||
|
Low |
Intermediate |
High |
|
|
Low |
18 |
77 |
20 |
|
Intermediate |
4 |
57 |
36 |
|
High |
0 |
16 |
12 |
Caption: RP = radical prostatectomy.
Concordance between pre- and post-RP recurrence risks was found in 75 patients (35.4%), upstaging in 4 (1.9%), and downstaging in 133 (62.7%). Among the 115 patients with low preoperative risk, 97 (84.3%) had an increased risk of recurrence after RP, 77 (79.4%) progressed to intermediary risk, and 20 (20.6%) to high risk. Among the 97 patients with intermediate preoperative risk, 36 were classified post-operatively as high-risk.
Figure 1 shows the average time from diagnosis to RP surgery, which was 176.1 ± 120.2 days, varying from 29 days to a maximum time of 798 days. In patients clinically classified as having low recurrence risk, this time was 187 ± 123.1 days, ranging from 31 to 798 days, and in those with intermediate risk, 163.2 ± 115.8 days, ranging from 29 to 602 days.
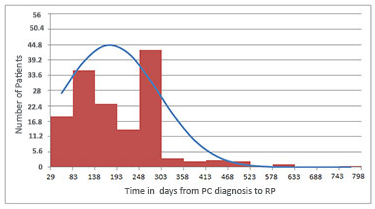
Figure 1. Time from prostate cancer diagnosis to operation in 212 patients treated with RP
Captions: PC = prostate cancer; RP = radical prostatectomy.
The Kaplan-Meier survival curve, plotted with 212 patients, indicates that the longer the delay from diagnosis to operation, more upstaged cancer is found (Figure 2). Patients who underwent surgery in up to 60 days showed approximately 95% probability of no increase in initial recurrence risk, decreasing to 80%, 70% and 50%, when undergoing operations within 100, 120, and 180 days, respectively.
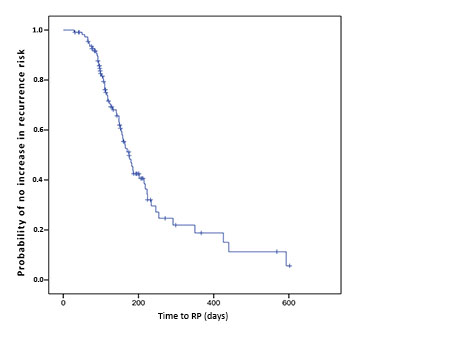
Figure 2. Probability of no increase in recurrence risk of prostate cancer plotted against time from neoplasia diagnosis to RP
DISCUSSION
The populational results encountered concur with the literature, with predominance of low-risk patients13,14. The study design did not allow the evaluation of the influence of time on disease progression in high-risk patients, who were excluded from the analysis. Nevertheless, Graefen et al., on analyzing high risk patients, observed that there was no statistical significance in biochemical recurrence rates among men who underwent early RP (<31 days after diagnosis) and those who underwent late RP (<70 days after diagnosis)9. For such patients, Moul et al.15 reported that there was no clear evident global impact regarding time from diagnosis to RP.
Among the 212 low risk and intermediate risk patients, there was concordance between clinical and pathological staging in about one-third of the cases and a predominance of subjects with recurrence risk progression. Alonso et al.2 suggest that these divergences are likely more related to errors in preoperative staging than to disease progression. They attribute them to factors related to biopsy sample failure and inter- and intra-observational variability, and argue that there is no correlation between wait time to surgery and upstaging2. The present paper challenges Alonso et al.2’s arguments in that the authors identified a time curve related to upstaging (Figure 2).
Graefen et al.9 found time gaps of 5 to 518 days between diagnosis and operation, mean of 62 days, and median 54 days9. Most international studies limited their investigation to assessing the impact of prolonged delays on disease progression and biochemical recurrence risk, and failed to estimate a reasonably safe time delay that could minimize poorer prognostic outcomes. In developed countries, sample sizes of patients in a prolonged-wait situation are quite small. That said, Graefen et al.9 and Freeland et al.16, due to the small number of patients who waited more than 6 months, were unable to assess the effect of long delay before treatment.
In the present study, average and maximum delay to RP times were longer than those described in the literature. This difference in relation to other studies mirrors the unfavorable treatment care conditions confronted in the Brazilian public health care system. This investigation purports to provide an evidence basis for reducing diagnosis-to-treatment time, and consequently, to enable the estimation of goals for safe surgical treatment outcomes.
For the study patients undergoing surgery within 60 days, the probability of prognosis worsening is less than 5%. Other studies report similar findings: prolonged delays (over 6 months) are related to significantly higher biochemical recurrence risk and lower progression-free survival13,16,17. Nam et al.8, in a study of 645 males, justify the increase as being the result of micrometastatic growth during long waits, even in the absence of primary tumor growth8. Other authors noted that patients whose surgical treatment delay was equal to or greater than 18 months were more prone to presenting higher Gleason scores for the pathological specimen18. However, no study has been able to establish a safe time for performing RP (Figure 2).
Although patients upon receiving a diagnosis of prostate cancer often wish to schedule their RP operation immediately, in Brazil, rapid and timely access to surgical care is often not available – a situation that can lead to the development of potentially adverse effects among those treated in the public health system.
In recent years, the distribution of the first treatment given to patients with prostate cancer, according to the stage of the disease, presents a pattern with small changes. According to Law number 12,732, of November 22, 2012, “the patient with malignant neoplasm has the right to undergo the first treatment at the National Health System (SUS), within a period of up to 60 (sixty) days counting from the day on which the diagnosis is confirmed in the pathological report or within a shorter period of time, depending on the therapeutic need of the case recorded in a single medical record”.
An analysis of the historical series from 2012, the year in which the 60-day law was enacted, shows that the aforementioned law did not have any impact on reducing the time between the diagnosis of prostate cancer and the first treatment19. Even for the most serious cases, the initiation of treatment has not been prioritized. Only 52.4% of patients with early-stage cancer and 58.0% with advanced cancer had access to treatment within 60 days of diagnosis. The definition of an optimal time to operation – without delay – remains challenging in prostate cancer treatment.
It is still unclear whether prostate cancer screening is capable of bringing any benefit to men and, if it does bring any benefit, whether it would be greater than the risks found20,21. The introduction of screening programs is based on the possible benefits in reducing mortality and morbidity by identifying the disease in its early stages when the prognosis is more favorable. However, in the case of prostate cancer, there is no consistency between clinical trials and meta-analyses regarding the real existence of a difference in mortality when comparing groups of screened and non-screened men. However, it is extremely necessary to organize the assistance, and secure access to men with urinary signs and symptoms in primary care so that the diagnostic investigation process can be started as soon as possible. Furthermore, it is essential to ensure referral to secondary units for diagnostic confirmation of suspected cases identified in primary care20,21. The results show a worsening of cancer staging the longer it takes between diagnosis and surgery (treatment).
The study has some limitations such as the small number of patients, a short follow-up time and, in addition, patients at high risk were not included according to D`Amico et al.7 classification.
The results herein show that waiting affects prognosis negatively, hence, every effort should be made to perform RP rapidly. Further research is needed to separately assess clinical staging in low and intermediate risk patients in light of new technologies, especially magnetic resonance imaging. It is also necessary to identify the major factors responsible for time-delay-to-RP to support and inform public policies focused on expedited treatment of prostate cancer.
CONCLUSION
The results show a worsening of cancer staging as the time between diagnosis and surgery increases. Patients operated within 60 days had a probability of no worsening of the risk of initial recurrence of approximately 95%, which decreased to 80%, 70% and 50% when operated within 100, 120 and 180 days, respectively. Delay in performing RP reflects the ongoing risk of worsening the staging of the neoplasm.
Safe delay time from prostate cancer diagnosis to RP cannot be determined as a definitive numerical figure. Considering that delay until surgery puts patients at ongoing risk of cancer upstaging, an optimal delay time may be considered as within 60 days of prostate biopsy – a period in which the probability of cancer upstaging is less than 5%.
CONTRIBUTIONS
All the authors contributed to the study design, data analysis, wording and approved the final version for publication.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
FUNDING SOURCES
None.
REFERENCES
1. Santos MO, Lima FCS, Martins LFL, et al. Estimativa de incidência de câncer no Brasil, 2023-2025. Rev Bras Cancerol. 2023;69(1):e-213700. doi: https://doi.org/10.32635/2176-9745.RBC.2023v69n1.3700
2. Alonso AR, Blanco AF, Fernández SP, et al. Influencia de la demora quirúrgica en los hallazgos patológicos y el pronóstico de los pacientes con cáncer de próstata. Actas Urol Esp. 2009;33(10):1069-77.
3. Antonopoulos IM, Pompeo ACL, Saldanha LB, et al. Gleason score comparative study between transrectal prostate biopsy and radical prostatectomy specimen. Int Braz J Urol. 2000;26(6):609-13.
4. Grossfeld GD, Chang JJ, Broering JM, et al. Under staging and under grading in a contemporary series of patients undergoing radical prostatectomy results from the cancer of the prostate strategic urologic research endeavor database. J Urol. 2001;165(3):851-6.
5. Noguchi M, Stamey TA, McNeal JE, et al. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. J Urol. 2001;166(1):104-10.
6. Obek C, Louis P, Civantos F, et al. Comparison of digital rectal examination and biopsy results with the radical prostatectomy specimen. J Urol. 1999; 161(2):494-8.
7. D'Amico AV, Whittington R, Malkowicz SB, et al. Clinical utility of percent-positive prostate biopsies in predicting biochemical outcome after radical prostatectomy or external-beam radiation therapy for patients with clinically localized prostate cancer. Mol Urol. 2000;4(3):171-5.
8. Nam RK, Jewett MA, Krahn MD, et al. Delay in surgical therapy for clinically localized cancer and biochemical recurrence after radical prostatectomy. Can J Urol. 2003;10(3):1891-8.
9. Graefen M, Walz J, Chun KHF, et al. Reasonable delay of surgical treatment in men with localized prostate cancer - impact on prognosis? Eur Urol. 2005;47(6):756-60. doi: https://doi.org/10.1016/j.eururo.2005.02.004
10. Korets R, Seager CM, Pitman MS, et al. Effect of delaying surgery on radical prostatectomy outcomes: a contemporary analysis. BJU International. 2012;110(2):211-6. doi: https://doi.org/10.1111/j.1464-410x.2011.10666.x
11.Sean Ong XR, Condon B, Bagguley D, et al. Safety first: evidence for delay of radical prostatectomy without use of androgen deprivation therapy during Covid-19. Future Oncol. 2020;16(20):1409-11.
13. O’Brien D, Loeb S, Carvalhal GF, et al. Delay of surgery in men with low risk prostate cancer. J Urol. 2011;185(6):2143-7. doi: https://doi.org/10.1016/j.juro.2011.02.009
14. Singer EA, Kaushal A, Turkbey B, et al. Active surveillance for prostate cancer: past, present and future. Curr Opin Oncol. 2012;24(3):243-50. doi: https://doi.org/10.1097%2FCCO.0b013e3283527f99
15. Moul JW, Leon S, Amling CL. How long can radical prostatectomy (RP) safely be delayed? CPDR’s experiences from 3324 cases. J Urol. 2004;171(4 sup):312.
16. Freedland SJ, Kane CJ, Amling CL, et al. Delay of radical prostatectomy and risk of biochemical progression in men with low risk prostate cancer. J Urol. 2006;175(4): 1298-303.
17. Holmstrom B, Holmberg E, Egevad L, et al. Outcome of primary versus deferred radical prostatectomy in the National Prostate Cancer Register of Sweden Follow-up. Study J Urol. 2010;184(4):1322-7.
18. O’Kelly F, Thomas A, Murray D, et al. Can delayed time to referral to a tertiary level urologist with an abnormal PSA level affect subsequent Gleason grade in the opportunistically screened population? The Prostate. 2013;73(12):1263-9.
19. Instituto Vencer o Câncer. Projeto de investigação sobre o cenário do câncer de próstata no sistema de saúde público brasileiro. Leitura estratégica integrada: perfil epidemiológico e políticas públicas. São Paulo: Instituto Vencer o Câncer; 2020. [Acesso 2023 jun 15]. Disponível em: https://vencerocancer.org.br/wp-content/uploads/2020/12/LEI_InstitutoVencer_PDF_Interativo_V4.pdf
20. Instituto Nacional de Câncer. Monitoramento das ações de controle do câncer de próstata. Bolet Inf detecção precoce. 2014;5(2):1-8.
21. Santos ROM, Abreu MM, Migowski A, et al. Ferramenta de apoio à decisão sobre o rastreamento do câncer de próstata no Brasil. Rev saúde pública. 2022;56:19. doi: https://doi.org/10.11606/s1518-8787.2022056003467
Recebido em 25/09/2023
Aprovado em 19/01/2024
Scientific-editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.