ORIGINAL ARTICLE
Predictive Power of Demographic and Clinicopathological Aspects of Patients with Acral Melanoma: a Single-Center Series Of 394 Cases in Brazil
Poder Preditivo dos Aspectos Demográficos e Clinicopatológicos de Pacientes com Melanoma Acral: uma Série de 394 Casos em um Único Centro no Brasil
Poder Predictivo de Aspectos Demográficos y Clinicopatológicos en Pacientes con Melanoma Acral: una Serie de 394 Casos en un Solo Centro en el Brasil
https://doi.org/10.32635/2176-9745.RBC.2024v70n1.4412
Luiz Fernando Nunes1; Lívia Costa de Oliveira2; Gélcio Luiz Quintella Mendes3; Alberto Julius Alves Wainstain4; Luiz Claudio Santos Thuler5; Anke Bergmann6
1-3,5,6Instituto Nacional de Câncer (INCA). Rio de Janeiro (RJ), Brazil. E-mails: lfernandonunes@gmail.com; lillycostaoliveira@gmail.com; gelciomendes@hotmail.com; lthuler@inca.gov.br; abergmann@inca.gov.br. Orcid iD: https://orcid.org/0000-0003-2835-6597; Orcid iD: https://orcid.org/0000-0002-5052-1846; Orcid iD: https://orcid.org/0000-0001-9564-0892; Orcid iD: https://orcid.org/0000-0003-2550-6537; Orcid iD: https://orcid.org/0000-0002-1972-8777
4Faculdade de Ciências Médicas de Minas Gerais (FCM-MG). Belo Horizonte (MG), Brazil. E-mail: albertojaw@gmail.com. Orcid iD: https://orcid.org/0000-0002-8227-7972
Corresponding author: Luiz Fernando Nunes. Rua General Ribeiro da Costa, 137, apto. 402 – Leme. Rio de Janeiro (RJ), Brazil. CEP 22010-050. E-mail: lfernandonunes@gmail.com
ABSTRACT
Introduction: Acral melanoma (AM) is associated with high mortality and poor survival, and its prognosis is worse compared to other melanoma subtypes. Objective: To analyze the predictive power of demographic and clinicopathological aspects in patients with AM. Method: This is a retrospective study with patients diagnosed with AM between January 2001 and December 2015. Demographic and clinicopathological characteristics were collected. The outcome was 5-year overall survival (OS). Kaplan-Meier curves, log rank-test and Cox regression analysis were used. Results: The study identified 394 patients with AM. The 5-year survival rate for patients with AM was found to be 45.6%. The predictive factors of OS included Breslow thickness [hazard ratio (HR): 1.02, 95% confidence interval (CI): 1.01-1.03], ulceration (HR: 4.06, 95%CI: 2.18-7.57) and lymphovascular invasion (LVI) (HR: 2.12, 95%CI:1.12-4.00). Conclusion: The findings highlight the poor prognosis of AM and the predictive power of Breslow thickness, ulceration and LVI.
Key words: melanoma; hand-foot syndrome; survival; prognosis.
RESUMO
Introdução: O melanoma acral (MA) está associado à alta mortalidade e à baixa sobrevida, e seu prognóstico é pior em comparação com os outros subtipos de melanoma. Objetivo: Analisar o poder preditivo de aspectos demográficos e clinicopatológicos em pacientes com MA. Método: Estudo retrospectivo com pacientes diagnosticados com MA entre janeiro de 2001 e dezembro de 2015. Foram coletadas características demográficas e clinicopatológicas. O desfecho foi a sobrevida global (SG) em cinco anos. Foram utilizados curvas de Kaplan-Meier, teste de log-rank e análise de regressão de Cox. Resultados: Foram identificados 394 pacientes com MA. A taxa de sobrevida em cinco anos para pacientes com MA foi de 45,6%. Os fatores preditivos da SG incluíram espessura de Breslow [hazard ratio (HR): 1,02, intervalo de confiança (IC) de 95%: 1,01-1,03], ulceração (HR: 4,06, IC 95%: 2,18-7,57) e invasão linfovascular (ILV) (HR: 2,12, IC 95%: 1,12-4,00). Conclusão: Tais achados destacam o prognóstico desfavorável do MA e o poder preditivo da espessura de Breslow, ulceração e ILV.
Palavras-chave: melanoma; síndrome mão-pé; sobrevida; prognóstico.
RESUMEN
Introducción: El melanoma acral (MA) está asociado con una alta mortalidad y una baja supervivencia, y su pronóstico es peor en comparación con los otros subtipos de melanoma. Objetivo: Analizar el poder predictivo de los aspectos demográficos y clinicopatológicos en pacientes con MA. Método: Estudio retrospectivo con pacientes diagnosticados con MA entre enero de 2001 y diciembre de 2015. Se recopilaron características demográficas y clinicopatológicas. El resultado fue la supervivencia global (SG) a los cinco años. Se utilizaron curvas de Kaplan-Meier, prueba de log-rank y análisis de regresión de Cox. Resultados: Se identificaron 394 pacientes con MA. La tasa de supervivencia a cinco años para los pacientes con MA fue del 45.6%. Los factores predictivos de la SG incluyeron el grosor de Breslow [razón de peligro (HR): 1.02, intervalo de confianza del 95% (IC): 1.01-1.03], la ulceración (HR: 4.06, IC del 95%: 2.18-7.57) y la invasión linfovascular (ILV) (HR: 2.12, IC del 95%: 1.12-4.00). Conclusión: Estos hallazgos resaltan el pronóstico desfavorable del MA y el poder predictivo del grosor de Breslow, la ulceración y la ILV.
Palabras clave: melanoma; síndrome mano-pie; sobrevida; pronóstico.
INTRODUCTION
Malignant melanoma, characterized by its aggressive nature, has exhibited a notable increase in incidence rates in recent times. In the United States of America, there were an estimate of 96,480 new cases of invasive melanoma diagnosed in 2019, resulting in 7,230 deaths1. Acral melanoma (AM) was described by Reed2, and it represents one of the four major subtypes. The age-adjusted incidence rate for AM was 1.8 per 1,000,000 person-years3 according to the Surveillance, Epidemiology, and End Results (SEER) 17 cancer registries. Prior data indicate substantial variations in anatomical origin, clinical characteristics, and prognoses among different ethnic groups3-6. The underlying causes of its pathogenesis remain unclear. Suspected risk factors for the incidence and spread of AM include chemical exposure, traumatic injury, prolonged physical stress, and pressure strength7-9.
AM is associated with elevated mortality rates and poor survival, with a prognosis notably worse when compared to other melanoma subtypes3,10-12. Due to the advanced stage at the time of presentation, several studies have postulated that the diminished survival can largely be attributed to delayed diagnosis13,14. An alternative, and not necessarily conflicting hypothesis suggests that AM may constitute a biologically more aggressive subtype of melanoma. In such a scenario, even early-stage disease could result in a poorer outcome. A study conducted by Bradford et al3. compared patients with AM and non-acral cutaneous melanoma (NACM) using data from SEER database. They discovered that when adjusting to the American Joint Commission on Cancer (AJCC) stage, AM patients exhibited lower survival rates in both stage II and stage III disease3,14.
The understanding of the characteristics and prognosis of AM remains limited, and there is still a substantial knowledge gap in Brazil regarding comprehensive clinical research on AM. This article presents a substantial Brazilian single-institution series of 394 patients diagnosed with AM, aiming to identify demographic and clinicopathological factors associated with overall survival (OS). In this context, investigating the prognostic factors of OS in the AM population can provide valuable insights into the course and characteristics of the disease. Thus, the objective of this study is to analyze the predictive power of demographic and clinicopathological aspects in patients with AM.
METHOD
Conducted as a retrospective hospital cohort study at an oncology referral center in Rio de Janeiro, the study focuses on the treatment of patients with AM, defined by the anatomic site of the melanoma, specifically focusing on the palmar, plantar, or subungual areas. Eligible participants included patients diagnosed with AM between January 1, 2001, and December 31, 2015, with histopathological confirmation. Patient data was obtained from the Division of Pathology database.
A total of 504 cases were identified during the study period. For cases initially evaluated at another hospital where the biopsy was conducted, slides and paraffin blocks, if available, were reviewed by the Division of Pathology. However, 110 patients were excluded from the analysis for various reasons: 2 were under 18 years of age, 13 were diagnosed with other synchronous or metachronic neoplasms, 39 did not undergo surgical treatment, 59 did not have information on inflammatory biomarkers, and 5 had melanoma "in situ".
The following demographic variables were collected: age at diagnosis (presented both as a continuous variable and categorized as <60 vs. >60 years old); sex (male vs. female); and Fitzpatrick skin type (I – pale skin, blue/green eyes, blond/red hair, always burns, does not tan; II – fair skin, blue eyes, burns easily, tans poorly; III – moderately fair skin, tans after initial burn; IV – light brown skin, burns minimally, tans easily; V – brown skin, rarely burns, tans darkly easily; VI – dark brown or black skin, never burns, always tans darkly)15.
Clinical and pathological variables were also collected: the anatomical location (plantar lesion vs. palmar vs. subungual), the histological type (superficial spread melanoma vs. melanoma nodular vs. acrolentiginous melanoma), the thickness of the melanoma was measured in millimeters (mm) as recommended by Breslow16, (presented continuously, and as a categorical variable (T), “T1” ≤ 1.0mm vs. “T2” – > 1.0 – 2.0 vs. “T3” - > 2.0 – 4.0 vs. “T4” - > 4.0mm). The level of invasion was also presented categorically as proposed by Clark17, ranging from I to V.
Microscopic ulceration was presented dichotomously as present vs. absent, as well as the variable mitosis, lymphovascular and perineural invasion, regression and intratumoral lymphocytic infiltration. The stage at diagnosis was categorized in accordance with the 2018 AJCC guidelines as stages I, II, III, and IV18,19 and the sentinel lymph node biopsy (SLNB) was classified as “positive”, when lymph node metastasis was present vs. “negative” if not present . For assessing long-term survival outcomes, OS was adopted and calculated from the date of AM diagnosis to either the date of death from any cause or the most recent follow-up for surviving patients. OS was measured in months.
For statistical analysis, the SPSS software version 23.0.0 (Statistical Package for Social Science for Windows, Inc., USA) was utilized and p value < 0.05 was considered statistically significant.
Proportions were compared utilizing the chi-squared test. To evaluate survival probabilities based on variable categories, Kaplan-Meier curves and the log-rank test were calculated. The Cox proportional hazard model was utilized, with hazard ratio (HR) and 95% confidence intervals (CI) serving as effect measures. Variables demonstrating p < 0.20 in the univariate analysis were included in the multiple regression analysis using the stepwise method, and those with p value < 0.05 were retained in the model.
The Institutional Review Board (IRB) of the institution (CAAE (submission for ethical review) 40068514.9.0000.5274), report 3286340 approved the study, in compliance with Resolution 466/201220 of the National Health Council and Good Clinical Practices guidelines.
RESULTS
The clinical and pathological characteristics of the AM cohort (n = 394) are presented in Table 1. Most of the patients were females (58.1%) with >60 years old (69.5%). Fitzpatrick skin phototypes I and II accounted for 52.8% of the cohort. Among the 394 patients, 70.1% had plantar lesions, 2.0% had palmar lesions, 27.1% had subungual lesions of the foot, and 0.8% had unreported information. The median thickness of the melanomas was 6.0 mm, with the majority of the patients categorized as Clark's level IV (37.6%) or V (33.8%) melanomas. At the diagnosis, most of the females was classified at stages II (45.2%) and III (37.8%).
The proportion of death was higher in AM patients with thicker tumors (T4) (69.5% vs 46.6%; p < 0.001), in ulcerated lesions (85.2% vs. 57.1%; p < 0.001), in the presence of mitosis (64.0% vs. 47.6%; p = 0.029) and with lymphovascular invasion (22.2% vs. 12.0%; p < 0.001) and it was statistically significative (Table 1).
|
Table 1. Demographic and clinicopathological characteristics of patients diagnosed with acral melanoma and death in 5-year stratification, between 2001 and 2015 (N=394)
|
||||
|
Variables |
N (%) |
Death (in 5-year) |
p value* |
|
|
Yes N (%) |
No N (%) |
|||
|
Age at diagnosis (years) |
|
|
|
0.737 |
|
<60 |
120 (30.5) |
68 (49.6) |
69 (50.4) |
|
|
≥60 |
274 (69.5) |
123 (47.9) |
134 (52.1) |
|
|
Sex |
|
|
|
0.153 |
|
Male |
165 (41.9) |
73 (44.2) |
92 (55.8) |
|
|
Female |
229 (58.1) |
118 (51.5) |
111 (48.5) |
|
|
Fitzpatrick skin type |
|
|
|
0.429 |
|
I/II |
208 (52.7) |
99 (47.6) |
109 (52.4) |
|
|
III/IV |
92 (23.4) |
50 (56.2) |
39 (43.8) |
|
|
V/VI |
89 (22.6) |
51 (55.4) |
41 (44.6) |
|
|
Unknown |
5 (1.3) |
3 (60.0) |
2 (40.0) |
|
|
Anatomical location |
|
|
|
0.665 |
|
Plantar |
276 (70.1) |
148 (53.6) |
128 (46.4) |
|
|
Palmar |
8 (2.0) |
3 (37.5) |
5 (62.5) |
|
|
Subungual |
107 (27.1) |
50 (46.4) |
57 (53.6) |
|
|
Unknown |
3 (0.8) |
2 (66.7) |
1 (33.3) |
|
|
Breslow (T) |
|
|
|
< 0.001 |
|
T1 |
28 (7.1) |
6 (21.4) |
22 (78.6) |
|
|
T2 |
40 (10.2) |
12 (30.0) |
28 (70.0) |
|
|
T3 |
80 (20.2) |
36 (45.0) |
44 (55.0) |
|
|
T4 |
230 (58.4) |
141 (61.3) |
89 (38.7) |
|
|
Unknown |
16 (4.1) |
8 (50.0) |
8 (50.0) |
|
|
Ulceration |
|
|
|
< 0.001 |
|
No |
74 (18.8) |
17 (23.0) |
57 (77.0) |
|
|
Yes |
282 (71.6) |
173 (61.3) |
109 (38.7) |
|
|
Unknown |
38 (9.6) |
13 (34.2) |
25 (65.8) |
|
|
Mitosis |
|
|
|
< 0.001 |
|
No |
38 (9.6) |
8 (21.1) |
30 (78.9) |
|
|
Yes |
221 (56.1) |
130 (58.8) |
91 (41.2) |
|
|
Unknown |
135 (34.3) |
65 (48.1) |
70 (51.9) |
|
|
Lymphovascular invasion |
|
|
|
0.029 |
|
No |
199 (50.5) |
96 (48.2) |
103 (51.8) |
|
|
Yes |
68 (17.3) |
45 (66.2) |
23 (33.8) |
|
|
Unknown |
127 (32.2) |
62 (48.8) |
65 (51.2) |
|
|
Caption: N = number of observations. (*) chi-square test for proportions. |
||||
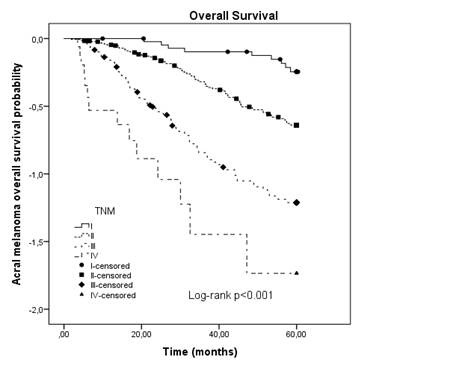
The Cox proportional hazard models are shown in Table 2. On univariate analysis, Breslow thickness (p = 0.005), ulceration (p < 0.001), mitosis (p < 0.001), lymphovascular invasion (LVI) (p < 0.032) and SLN positivity (p = 0.029) were all associated with reduced OS. The multiple model demonstrated that Breslow thickness [HR: 1.02 (95% CI: 1.01-1.03)], ulceration [HR: 4.06 (95% CI: 2.18-7.57)] and LVI [HR: 2.12 (95% CI: 1.12-4.00)] are predictive factors of 5-year OS. The 5-year OS rate for all primary AM by TNM AJCC, 2018 classification was (I – 78.2%, II – 53.1, III – 29.7 and IV – 17.6). The median follow-up was 41.1 months (range 0-60). The median OS was 44.4 months (range 1.9-60). The AM 5-year survival rate was 45.6%. The Kaplan-Meier OS curve for patients with AM stratified by AJCC, 2018, showed survival differences between stages and it was statistically significant (p < 0.001) (Figure 1).
|
Table 2. Predictive power of demographic and clinical-pathological factors in 5-year overall survival in acral melanoma cohort, between 2001 and 2015 (N= 394)
|
||||
|
Variables |
Univariate (HR CI 95%) |
p value |
Multivariate (HR CI 95%) |
p value |
|
Age at diagnosis (years) |
1.00 (0.99-1.02) |
0.378 |
|
|
|
Sex |
|
0.154 |
|
|
|
Female |
1.00 |
|
|
|
|
Male |
1.34 (0.90-2.00) |
|
|
|
|
Fitzpatrick skin cancer |
|
0.431 |
|
|
|
I/II |
1.00 |
|
|
|
|
III/IV |
1.41 (0.86-2.33) |
|
|
|
|
V/VI |
1.37 (0.84-2.24) |
|
|
|
|
Unknown |
1.65 (0.27-10.09) |
|
|
|
|
Anatomical location |
|
0.670 |
|
|
|
Plantar |
1.00 |
|
|
|
|
Palmar |
0.52 (0.12-2.21) |
|
|
|
|
Subungual hand |
0.77 (0.39-1.54) |
|
|
|
|
Subungual foot |
0.75 (0.44-1.27) |
|
|
|
|
Unknown |
1.73 (0.16-19.30) |
|
|
|
|
Breslow thickness (mm) |
1.02 (1.01-1.03) |
0.005 |
1.01 (1.01-1.02) |
0.027 |
|
Ulceration |
|
< 0.001 |
|
< 0.001 |
|
No |
1.00 |
|
1.00 |
|
|
Yes |
5.32 (2.94-962) |
|
4.06 (2.18-7.57) |
|
|
Unknown |
1.74 (0.74-4.13) |
|
1.13 (0.44-2.91) |
|
|
Mitosis |
|
< 0.001 |
|
|
|
No |
1.00 |
|
|
|
|
Yes |
5.36 (2.35-12.22) |
|
|
|
|
Unknown |
3.48 (1.49-8.15) |
|
|
|
|
Lymphovascular invasion |
|
0.032 |
|
0.021 |
|
No |
1.00 |
|
1.00 |
|
|
Yes |
2.10 (1.18-3.73) |
|
2.12(1.12-4.00) |
|
|
Unknown |
1.02 (0.66-1.60) |
|
0.98(0.58-1.67) |
|
|
TIL |
|
0.539 |
|
|
|
No |
1.00 |
|
|
|
|
Brisk |
1.46 (0.64-3.31) |
|
|
|
|
Non-brisk |
0.46 (0.04-4.97) |
|
|
|
|
Unknown |
1.59 (0.68-3.73) |
|
|
|
|
SLNB |
|
0.029 |
|
|
|
Negative |
1.00 |
|
|
|
|
Positive |
1.98 (1.07-3.65) |
|
|
|
|
Captions: HR = hazard ratio; CI = confidence interval; TIL = tumour infiltrating lymphocyte; SLNB = sentinel lymph node biopsy. |
||||
|
|
|
Source: adapted18,19.
|
The Kaplan-Meier OS curves for patients with AM and the presence of ulceration, LVI, and thick melanoma exhibited remarkable survival disparities, and these differences were statistically significant (p < 0.001) (Figure 2A, 2B, 2C). AM patients who underwent a SLN biopsy and had a positive SLN had a 47.3 % (95 % CI 37.9-47.4 %) 5-year OS compared with an 64.0 % (95 % CI 49.4-54.2 %) 5-year OS in those AM patients with a negative SLN. The Kaplan-Meier OS curve for patients with AM who were submitted to SLNB (n = 192) showed survival differences and it was statistically significant (p = 0.005) (Figure 2D).
|
|
|
Figure 2. Kaplan-Meier overall survival curve for acral melanoma stratified by (A) Lymphovascular invasion, (B) Ulceration, (C) Breslow’s depth (T) and (D) Sentinel lymph node in patients diagnosed with acral melanoma, between 2001 and 2015 (N=394) Source: adapted18,19. |
DISCUSSION
This retrospective multicenter cohort study offered evidence of the prognostic and clinical significance of Breslow thickness, ulceration and LVI in AM. The presence of ulceration at the primary tumor was associated with over a twofold increase in the risk of death from melanoma. Furthermore, the results indicated that a positive SLNB might aid clinicians in identifying patients who could potentially benefit from adjuvant systemic therapy and more intensive surveillance.
This study comprises a patient series from a single institution in Rio de Janeiro, Brazil. The characteristics of the patient group analyzed closely align with those observed in comparable investigations. The male-to-female ratio of 1:1.38 corresponds with findings reported by other authors21,22. In previous studies, no sex difference in AM was detected. The mean age (65.3 years) is quite similar to most of the studies, where the mean age of patients with AM ranged from 57 to 68 years23,24.
Within this cohort, individuals with Fitzpatrick skin types V and VI make up 22.6% of the entire AM population, a higher percentage of these skin types than what is reported in other studies25. Similar to the current research, previous reports have shown that the majority of AM cases occur on plantar sites, with only a minority occurring on palmar sites12,26 and nail apparatus melanoma accounted for 27.1% of AM cases, a proportion consistent with findings of other studies27,28 indicating that approximately 30% to 35% of AM was subungual.
Numerous AM cases exhibited both considerable thickness and ulceration, aligning with the retrospective literature that characterizes acral tumors in a similar manner8,10,12,14. Just as previous studies have suggested, there could be a significant delay in the diagnosis or treatment of AM patients. Nevertheless, it's important to note that the current study does not explore this particular issue.
The clinicopathologic features of AM revealed a substantial ulceration rate (71.6%) and a notable proportion of Clark level IV and V (37.6% and 33.8%). These findings may help account for the lower 5-year OS rate (44.4%), especially given that the diagnosis of AM is frequently delayed, and tumor thickness tends to be greater when compared to superficial spreading melanoma (SSM) or lentigo malignant melanoma (LMM). The five-year survival rates for AM reported in China and Japan, where AM is the predominant subtype, were below 50%29,30. Due to limited awareness of melanoma, lesions are frequently misdiagnosed as benign, leading to delayed diagnoses.
Ulceration is recognized as a negative prognostic factor in melanoma31,32, often used in conjunction with tumor thickness to guide treatment decisions and predict outcomes. These factors are fundamental elements of the AJCC melanoma staging system18. Nevertheless, the precise prognostic significance of ulceration in AM remains a subject of debate. The presence of ulceration over a melanoma typically signifies a biologically more aggressive tumor, which correlates with an unfavorable prognosis21. The mechanism underlying ulceration, however, remains poorly understood. It is theorized that the necrosis of the epidermis may result from rapid tumor growth and the disruption of dermal vascular supply.
Nodal status, particularly that of the sentinel lymph node (SLN), has established itself as a significant prognostic factor for cutaneous melanomas33-35. Where increasing Breslow thickness, ulceration, and LVI proved to be significant factors influencing OS, univariate analysis revealed that SLN status was a predictive factor in AM patients who underwent SLN biopsy. While the study population did not reach a sufficient size to precisely match the incidence of SLN positivity based on clinical stage, there was a notably high rate of positive SLN (33.3%) observed in the entire cohort, consistent with findings of other studies36.
Despite significant advances in treatments since 2011, the treatment protocol remained consistent over the study period. However, the impact of diagnosis year variability on prognosis through exploratory analysis was not explored in the current analysis. Future investigations should further examine this issue to gain insights into the temporal impact on prognostic outcomes.
AM is a rare condition, and despite the relative size of the series analyzed, both the complete AM cohort and its subpopulations are small, which restricts the statistical power of the analysis which is one of the several limitations of the present investigation. Furthermore, because the series is derived from a single institution, where patients were referred for surgical or systemic treatment, there might be inherent selection bias.
CONCLUSION
The findings highlight the poor prognosis of AM, emphasizing the need for further research and understanding of this rare and aggressive form of melanoma. Several independent risk factors for OS, including Breslow thickness, ulceration and LVI have been identified. These factors can aid in predicting the prognosis of AM patients and guide treatment decisions. The five-year survival rate for primary AM patients was determined to be 45.6%, underscoring the challenges faced in managing this disease. Further studies are warranted to validate these findings and explore potential therapeutic strategies to improve outcomes for individuals with AM.
CONTRIBUTIONS
Luiz Fernando Nunes contributed substantially to the study design, data analysis, and wording of the manuscript. Lívia Costa de Oliveira, Gélcio Luiz Quintella Mendes and Luiz Claudio Santos Thuler contributed substantially to data interpretation and critical review. Alberto Julius Alves Wainstein contributed substantially to critical review. Anke Bergmann contributed substantially to the study design, data analysis and interpretation and wording of the manuscript. All the authors approved the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
The author Anke Bergmann who is the scientific-editor of INCA’s Revista Brasileira de Cancerologia declares potential conflict of interests. The other authors have no conflict of interests to declare.
FUNDING SOURCES
None.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Reed RJ. New concepts in surgical pathology of the skin. In: Hartmann W, Kay S, Reed RJ, editors. Histopatogy. New York: John Wiley & Sons; 1976.
3. Bradford PT, Goldstein AM, McMaster ML, et al. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986-2005. Arch Dermatol. 2009;145(4):427-34.
4. Bristow I, Bower C. Melanoma of the foot. Clin Podiatr Med Surg. 2016;33(3):409-22.
5. Markovic SN, Erickson LA, Rao RD, et al. Malignant melanoma in the 21st century, part 2: staging, prognosis, and treatment. Mayo Clin Proc. 2007;82(4):490-513.
6. Piliang MP. Acral lentiginous melanoma. Clin Lab Med. 2011;31(2):281-8.
7. Green A, McCredie M, MacKie R, et al. A case-control study of melanomas of the soles and palms (Australia and Scotland). Cancer Causes Control. 1999;10(1):21-5.
8. Phan A, Touzet S, Dalle S, et al. Acral lentiginous melanoma: histopathological prognostic features of 121 cases. Br J Dermatol. 2007;157(2):311-8.
9. Rolón PA, Kramárová E, Rolón HI, et al. Plantar melanoma: a case-control study in Paraguay. Cancer Causes Control. 1997;8(6):850-6.
10. Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol. 2000;143(2):275-80.
11. Pollack LA, Li J, Berkowitz Z, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65(5 Supl 1):S78-86.
12. Slingluff CL, Vollmer R, Seigler HF. Acral melanoma: a review of 185 patients with identification of prognostic variables. J Surg Oncol. 1990;45(2):91-8.
13. Nunes LF, Quintella Mendes GL, Koifman RJ. Acral melanoma: a retrospective cohort from the Brazilian National Cancer Institute (INCA). Melanoma Res. 2018;28(5):458-64.
14. O’Leary JA, Berend KR, Johnson JL, et al. Subungual melanoma. A review of 93 cases with identification of prognostic variables. Clin Orthop Relat Res. 2000;(378):206-12.
15. Fitzpatrick TB. Enigma of the pathogenesis of primary melanoma: changing incidence and mortality in Japan and the United States. J Invest Dermatol. 1989;92(5 Supl):234S-5S.
16. Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970;172(5):902-8.
17. Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29(3):705-27.
18. Gershenwald JE, Scolyer RA. Melanoma Staging: American Joint Committee on Cancer (AJCC) 8th Edition and Beyond. Ann Surg Oncol. 2018;25(8):2105-10.
19. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472-92.
20. Conselho Nacional de Saúde (BR). Resolução n° 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília, DF. 2013 jun 13; Seção I:59.
21. Bello DM, Chou JF, Panageas KS, et al. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol. 2013;20(11):3618-25.
22. Cascinelli N, Zurrida S, Galimberti V, et al. Acral lentiginous melanoma. A histological type without prognostic significance. J Dermatol Surg Oncol. 1994;20(12):817-22.
23. Fortin PT, Freiberg AA, Rees R, et al. Malignant melanoma of the foot and ankle. J Bone Joint Surg Am. 1995;77(9):1396-403.
24. Scrivner D, Oxenhandler RW, Lopez M, et al. Plantar lentiginous melanoma. A clinicopathologic study. Cancer. 1987;60(10):2502-9.
25. Collins KK, Fields RC, Baptiste D, et al. Racial differences in survival after surgical treatment for melanoma. Ann Surg Oncol. 2011;18(10):2925-36.
26. Krementz ET, Feed RJ, Coleman WP, et al. Acral lentiginous melanoma. a clinicopathologic entity. Ann Surg. 1982;195(5):632-45.
27. Finley RK, Driscoll DL, Blumenson LE, et al. Subungual melanoma: an eighteen-year review. Surgery. 1994;116(1):96-100.
28. Kato T, Suetake T, Tabata N, et al. Epidemiology and prognosis of plantar melanoma in 62 japanese patients over a 28-year period. Int J Dermatol. 1999;38(7):515-9.
29. Lv J, Dai B, Kong Y, et al. Acral Melanoma in Chinese: a clinicopathological and prognostic study of 142 cases. Sci Rep. 2016;6:31432.
30. Seiji M, Takematsu H, Hosokawa M, et al. Acral melanoma in Japan. J Invest Dermatol. 1983;80(Supl):56s-60s.
31. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-206.
32. Crompton JG, Gilbert E, Brady MS. Clinical implications of the eighth edition of the American Joint Committee on Cancer melanoma staging. J Surg Oncol. 2019;119(2):168-74.
33. Gershenwald JE, Ross MI. Sentinel-lymph-node biopsy for cutaneous melanoma. N Engl J Med. 2011;364(18):1738-45.
34. Maduekwe UN, Hornicek FJ, Springfield DS, et al. Role of sentinel lymph node biopsy in the staging of synovial, epithelioid, and clear cell sarcomas. Annals of Surgical Oncology. 2009;16(5):1356-63.
35. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370(7):599-609.
36. Egger ME, Gilbert JE, Burton AL, et al. Lymphovascular invasion as a prognostic factor in melanoma. Am Surg. 2011;77(8):992-7.
Recebido em 2/10/2023
Aprovado em 29/12/2023
Executive-editor: Letícia Casado. Orcid iD: https://orcid.org/0000-0001-5962-8765
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.
©2019 Revista Brasileira de Cancerologia | Instituto Nacional de Câncer | Ministério da Saúde