CASE REPORT
Metastatic Retroperitoneal Leiomyosarcoma: Case Report
Leiomiossarcoma Retroperitoneal Metastático: Relato de caso
https://doi.org/10.32635/2176-9745.RBC.2024v70n1.4592
Samya Hamad Mehanna1; Emily Karoline Araujo Nonato Dos Santos2; Julia Costa Linhares3; Izabele Maria Geri4; Renata Namie Yoshioka Kimura5; Teresa Cristina Cavalcanti6
1,2,4,5Faculdade Evangélica Mackenzie do Paraná (Fempar). Curitiba (PR), Brasil. E-mails: samyahm88@gmail.com; emilynonatoaraujo@gmail.com; izabelemariageri@gmail.com; rehnamie@gmail.com. Orcid iD: https://orcid.org/0000-0002-6636-1314; Orcid iD: https://orcid.org/0000-0001-6671-4493; Orcid iD: https://orcid.org/0000-0002-8911-6135; Orcid iD: https://orcid.org/0000-0002-4933-3637
3Universidade Federal do Paraná (UFPR). Curitiba (PR), Brasil. E-mail: juliaclinhares@yahoo.com.br. Orcid iD: https://orcid.org/0000-0002-2651-0610
6Hospital Universitário Mackenzie (HUEM), Departamento de Patologia. Curitiba (PR), Brasil. E-mail: tecava@yahool.com.br. Orcid iD: https://orcid.org/0000-0002-8555-6547
Corresponding Author: Emily Karoline Araujo Nonato Dos Santos. Rua Padre Anchieta, 2770 – Bigorrilho. Curitiba (PR), Brasil. CEP 80730-000. E-mail: emilynonatoaraujo@gmail.com
ABSTRACT
Introduction: Leiomyosarcomas (LMS) are rare malignant neoplasms originating in smooth muscle, more common in women in their fifth and sixth decades of life. Inherent characteristics of the retroperitoneum allow LMS in this location to reach substantial proportions and present symptoms only in more advanced stages. Case report: A 37-year-old woman sought medical attention in July 2020 due to the growth of a painful, fixed mass in the left hemiabdomen that appeared six months earlier. The patient denied urinary or gastrointestinal alterations but reported an 8 kg weight loss in the last month. Computed tomography identified a lobulated, heterogeneous formation in the left flank measuring 8.5 cm, along with hepatic and pulmonary nodules. Subsequently, surgical resection of the lesion, nephroureterectomy, and hepatic biopsy were performed, confirming the diagnosis of LMS through anatomopathological and immunohistochemical analysis. After unsuccessful adjuvant chemotherapy, she progressed to multiple metastases and is currently undergoing palliative treatment. Conclusion: Detecting and diagnosing retroperitoneal LMS are challenging. Awareness of their aggressiveness, especially in young patients, is crucial to ensure personalized and early interventions, thereby improving the prognosis.
Key words: Epithelial-Mesenchymal Transition; Leiomyosarcoma/diagnosis; Delayed Diagnosis
RESUMO
Introdução: Leiomiossarcomas (LMS) são neoplasias malignas raras originadas em músculo liso. São mais frequentes em mulheres na quinta e sextas décadas de vida. Características inerentes ao retroperitônio permitem que LMS nessa localização adquiram grandes proporções e apresentem sintomas apenas em estágios mais avançados. Relato do caso: Mulher, 37 anos, procurou o hospital em julho de 2020 por causa do crescimento de uma massa dolorosa e fixa em hemiabdome esquerdo com início há seis meses. A paciente negava alterações urinárias ou gastrointestinais, porém referia perda de oito kg no último mês. A tomografia computadorizada identificou uma formação lobulada de caráter heterogêneo em flanco esquerdo medindo 8,5 cm, além de nódulos hepáticos e pulmonares. Em seguida, foram realizadas ressecção cirúrgica da lesão, nefroureterectomia e biópsia hepática, confirmando o diagnóstico de LMS por análise anatomopatológica e imuno-histoquímica. Após quimioterapia adjuvante sem sucesso, progrediu com múltiplas metástases e está atualmente em tratamento paliativo. Conclusão: A detecção e o diagnóstico dos LMS retroperitoneais são desafiadores. A conscientização sobre sua agressividade, especialmente em pacientes jovens, é crucial para garantir intervenções personalizadas e precoces, melhorando o prognóstico.
Palavras-chave: Transição Epitelial-Mesenquimal; Leiomiossarcoma/diagnóstico; Diagnóstico Tardio.
RESUMEN
Introducción: Los leiomiosarcomas (LMS) son neoplasias malignas raras que se originan en el músculo liso. Son más frecuentes en mujeres en la quinta y sexta décadas de vida. Las características inherentes al retroperitoneo permiten que los LMS en esta ubicación adquieran grandes proporciones y presenten síntomas solo en etapas más avanzadas. Informe del caso: Mujer de 37 años acudió al hospital en julio de 2020 debido al crecimiento de una masa dolorosa y fija en el hemiabdomen izquierdo que había comenzado seis meses antes. La paciente negó alteraciones urinarias o gastrointestinales, pero reportó una pérdida de peso de ocho kilogramos en el último mes. La tomografía computarizada identificó una formación lobulada y heterogénea en el flanco izquierdo que medía 8,5 cm, junto con nódulos hepáticos y pulmonares. Posteriormente, se realizó la resección quirúrgica de la lesión, nefroureterectomía y biopsia hepática, confirmando el diagnóstico de LMS mediante análisis anatomopatológico e inmunohistoquímico. Después de una quimioterapia adyuvante sin éxito, progresó a múltiples metástasis y actualmente recibe tratamiento paliativo. Conclusión: La detección y diagnóstico de los LMS retroperitoneales son desafiantes. La conciencia sobre su agresividad, especialmente en pacientes jóvenes, es crucial para garantizar intervenciones personalizadas y tempranas, mejorando el pronóstico.
Palavras clave: Transición Epitelial-Mesenquimal; Leiomiosarcoma/diagnóstico; Diagnóstico Tardío.
INTRODUCTION
Sarcomas are malignant mesenchymal neoplasms representing less than 1% of cancers in adults1, and 12-69% of the cases affect the peritoneum, the most affected site. Among these, leiomyosarcoma is the second most prevalent type, with 28% of the diagnoses, affecting individuals aged 54 to 65 years predominantly women with a ratio of 5:12.
Due to the available space in the retroperitoneum, the neoplasm has ample room to grow, becoming symptomatic only in more advanced stages3. Symptoms are generally associated with invasion or mass effect, leading to intestinal obstruction, early satiety, lower limb edema, and abdominal pain, with an asymptomatic abdominal mass that is non-painful upon palpation, the most commonly observed clinical presentation4.
Leiomyosarcoma (LMS) develops from retroperitoneal smooth muscle cells or from cells in the walls of large peritoneal veins, presenting macroscopic appearance with gray, white, or brownish coloring, and may also cause hemorrhage, necrosis, and cystic changes when they reach large sizes. Generally, the mass has well-circumscribed margins, with regions of infiltration into adjacent tissues2,3.
Given these facts, the main benefit of this article is to contribute to the literature by presenting a case of metastatic retroperitoneal leiomyosarcoma in a 37-year-old female patient, describing radiological and microscopic aspects. A thorough and comprehensive case report can significantly aid healthcare professionals in making accurate and informed differential diagnoses.
The Institutional Review Board of “Faculdade Evangélica do Paraná” approved the study, report number 4.762.396, CAAE (ethical submission) number 46320521.5.0000.0103, in compliance with Ordinance 466/20125 of the National Health Council.
CASE REPORT
A 37-year-old female patient was admitted to the emergency room in July 2020 due to complaints of palpable mass growth, painful in the left hemiabdomen for the past 6 months. She denied urinary and gastrointestinal changes, but reported an 8 kg weight loss in the last month. On physical examination, an inelastic and fixed mass occupying a large portion of the left flank was identified. A computed tomography (CT) scan of the abdomen revealed a lobulated and heterogeneous formation in the left flank measuring 8.5 cm in the largest axis (Figure 1). The mass displaced the upper renal vessel and posterior ureter, leading to urethral and pelvicalyceal dilatation. Additionally, multiple pulmonary and hepatic micronodules were identified.
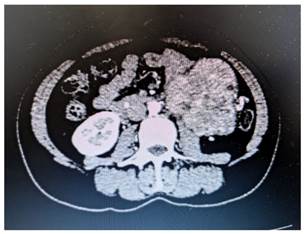
Figure 1. A – Abdominal CT showing the presence of a heterogeneous mass with regions of post-contrast enhancement, located in the left flank and measuring 8.5 x 8.5 x 8.0 cm.
Source: The authors, 2023
The patient underwent surgical excision of the tumor, left nephroureterectomy and liver biopsy. The histopathological report revealed malignant spindle cell sarcomatoid neoplasia, with marked cellularity, moderate cellular pleomorphism, significant mitotic index, and areas of necrosis, including demonstrated compression of the left ureter (Figure 2).
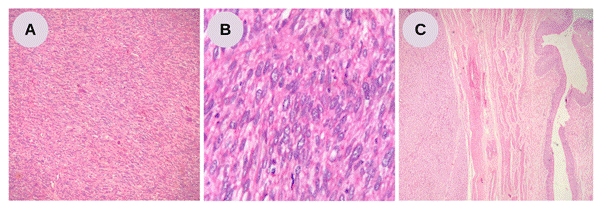
Figure 2. Retroperitoneal leiomyosarcoma. A – Neoplasm composed of spindle cells, with eosinophilic fibrillar cytoplasm arranged in long intersecting fascicles, parallel and perpendicular to the cutting plane (Optical Microscopy, Hematoxylin-eosin, 100x); B – Highlighting the moderate nuclear pleomorphism of the tumor cells (Optical Microscopy, Hematoxylin-eosin, 400x); C – Note the interface between the neoplasm on the left and the segment of the ureter on the right (Optical Microscopy, Hematoxylin-eosin, 40x). Source: The authors, 2023
The complementary immunohistochemical study of the neoplasm demonstrated positivity for smooth muscle actin, desmin (Figure 3), and caldesmon, markers of smooth muscle origin, corroborating the diagnosis of retroperitoneal leymiosarcoma. Microscopic analysis of the hepatic nodule confirmed metastasis of the same morphological pattern of the abdominal lesion.
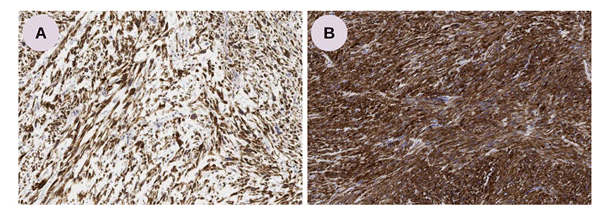
Figure 3. Retroperitoneal leiomyosarcoma. A- Positivity for desmin; B- Positivity for smooth muscle actin. (Optical Microscopy, Immunohistochemistry, 200x). Source: The authors, 2023
After surgical treatment, the patient underwent oncologic therapy, including six cycles of adjuvant chemotherapy with doxorubicin and ifosfamide, completed in March 2021. During the follow-up evaluation after the sixth cycle, the disease stabilized and there was a transition to gemcitabine and docetaxel completed in February 2022 leading to disease stabilization. However, one month later, there was worsening with growth of 33% of the liver lesion compared to the previous exam, prompting the adoption of 16 cycles of dacarbazine completed in July 2023. During this same period, bone metastases (in the spinal column and right humerus), lungs, and new hepatic nodules were identified. Currently, the patient is under palliative care and receives quarterly treatment with zoledronic acid.
DISCUSSION
Malignant stromal neoplasms characterized by cells exhibiting smooth muscle differentiation are referred to as leiomyosarcoma (LMS). Soft tissue LMSs, except those affecting solid organs, typically originate in the lower limbs, retroperitoneum, abdomen/pelvis, and trunk6. Another identifiable subgroup originates within major blood vessels, particularly in the inferior vena cava, its primary tributaries, and the major veins of the lower extremities7.
LMSs typically manifest as mass-like lesions and are often associated with nonspecific symptoms due to structural displacement rather than invasion. Symptoms related to LMS of the inferior vena cava vary based on their location. Obstruction of the hepatic veins in the upper portion can result in Budd-Chiari syndrome, characterized by hepatomegaly, jaundice, and ascites. Tumors in the middle portion may obstruct the renal veins, while involvement of the lower portion can lead to local edema. Although imaging studies such as MRI and contrast-enhanced CT lack specificity, they are invaluable for delineating the tumor's relationship with adjacent structures, particularly in the retroperitoneum8.
The incidence of soft tissue LMS increases with age, reaching its peak in the seventh decade of life, although it can also develop in younger adults and even children, representing approximately 11% of all recently diagnosed soft tissue sarcomas. Women are the majority of the patients with retroperitoneal and inferior vena cava LMS, but not among those with tumors located elsewhere. Identified predisposing factors contributing to its development encompass Li-Fraumeni syndrome, hereditary retinoblastoma, and exposure to radiation9.
In histology, the characteristic microscopic appearance of leiomyosarcoma are spindle cells forming fascicles with well-defined margins, and the presence of at least one of the following criteria for diagnosis: cellular pleomorphism or atypia, coagulative tumor necrosis, or over 10 mitotic figures per 50 high-power fields in women, and over 1 mitotic figure per 50 high-power fields in men2.
In the immunohistochemical analysis, at least one myogenic immunomarker such as smooth muscle actin, desmin, or h-caldesmon shows positivity in 100% of the cases, with over 70% demonstrating positivity for multiple markers. Since none of these markers are entirely specific for smooth muscle differentiation, positivity for two markers proves more conducive for an accurate diagnosis10. This analysis is of utmost importance, especially to distinguish among the main primary retroperitoneal sarcomas, which are well-differentiated liposarcoma, dedifferentiated liposarcoma, and leiomyosarcoma6.
In addition to histological and immunohistochemical criteria, computed tomography (CT) is considered the standard method for evaluating retroperitoneal leiomyosarcoma, allowing the analysis of disease location and possible metastases, as well as tumor-specific features such as necrosis and cystic changes1.
Surgical resection with tumor-free margins is considered the primary treatment for retroperitoneal sarcomas11, but due to the usually late diagnosis with tumor invasion into adjacent structures, this treatment can lead to recurrences, a common cause of mortality3. Thus, en bloc resection of adjacent organs is the recommended therapy according to the guidelines of the European Society for Medical Oncology for better prognosis11.
Although the literature highlights doxorubicin combined with ifosfamide as a key drug in the systematic treatment of retroperitoneal sarcomas (RPS), the patient did not respond well to this treatment, possibly due to genetic peculiarities of the tumor itself, which, unfortunately, were not tested. Among the mutations associated with an unfavorable response to this treatment are microsatellite mutations, TP53, and RB1. This underscores the importance of such investigation whenever possible before initiating adjuvant therapy12,13.
The adoption of the combination of gemcitabine and dacarbazine is one of the chemotherapy options that has shown positive results in LMS. However, other studies are necessary to determine increasingly targeted and effective treatments for this aggressive type of neoplasm14. Furthermore, the literature still diverges on the use of adjuvant and neoadjuvant chemotherapy and is currently considered experimental, with its use recommended to be individualized with authors suggesting its use only for patients with unresectable or metastatic disease12.
Furthermore, it is important to acknowledge the limitations associated with case reports. Due to the individualized nature of the study, generalizations to a wider patient population may be restricted. However, detailing a rare medical condition allows for valuable clinical and therapeutic insights into a rare and difficult-to-diagnose disease, providing crucial information about disease progression, response to different therapeutic modalities, and the challenges faced by healthcare professionals in managing these complex cases, particularly in the context of the National Health System (SUS). Additionally, it can serve as benchmark for the development of more effective treatment guidelines and clinical protocols for rare neoplasms15.
CONCLUSION
The detection of retroperitoneal leiomyosarcomas is challenging due to the rarity of the tumor in this location, as well as overlap with other local mesenchymal neoplasms, requiring histological and immunohistochemical analysis for accurate diagnosis. It is crucial to raise awareness within the medical community about this issue, given the potential aggressiveness of the disease in young patients. Thus, understanding this type of cancer is essential to ensure early and personalized treatments, which can significantly improve patients’ prognosis.
CONTRIBUTIONS
All authors contributed substantially to the study design, acquisition, analysis, and interpretation of the data, wording and critical review. They approved the final version to be published.
CONFLICT OF INTEREST
There is no conflict of interests to declare.
FUNDING SOURCES
None.
REFERENCES
1. McAddy NC, Hallin M, Strauss D, et al. CT imaging improves histopathological grading of retroperitoneal leiomyosarcomas. Eur J Surg Oncol. 2020;46(2):288-92. doi: https://doi.org/10.1016/j.ejso.2019.10.007
2. Marko J, Wolfman DJ. Retroperitoneal leiomyosarcoma from the radiologic pathology archives. Radiographics. 2018;38(5):1403-20. doi: https://doi.org/10.1148/rg.2018180006
3. Balti M, Ayari J, Ben Azaiez M, et al. Retroperitoneal leiomyosarcoma in adults. Presse Med. 2019;48:77-9. doi: https://doi.org/10.1016/j.lpm.2018.11.003
4. Oliveira DH, Lima FM, Ramos AR, et al. Sarcoma retroperitoneal: relato de caso. Rev Saber Dig. 2021;14(1):32. doi: https://doi.org/10.24859/SaberDigital.2021v14n1.935
5. Mack T, Purgina B. Updates in pathology for retroperitoneal soft tissue sarcoma. Current Oncology. 2022;29(9):6400-18. doi: https://doi.org/10.3390/curroncol29090504
6. Sacoto Urgilez D. Vascular Retroperitoneal and Pelvic Leiomyosarcoma a Rare Disease Requiring Complex Surgery. J Cancer Res Therap Oncol. 2020;8:1-5
7. World Health Organization. WHO Classification of Tumours editorial board. soft tissue and bone tumours. 5. ed. Lyon: IARC; 2020. v. 3.
8. Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110(6):649-60. doi: https://doi.org/10.1093/jnci/djx235
9. Ducimetière F, Lurkin A, Ranchère-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6(8):e20294. doi: https://doi.org/10.1371/journal.pone.0020294
10. Kirane A, Crago AM. The importance of surgical margins in retroperitoneal sarcoma. J Surg Oncol. 2016;113(3):270-6. doi: https://doi.org/10.1002/jso.24135
11. Sassa N. Retroperitoneal tumors: review of diagnosis and management. Int J Urol. 2020;27(12):1058-70. doi: https://doi.org/10.1111/iju.14361
12. Dominguez DA, Sampath S, Agulnik M, et al. Surgical Management of Retroperitoneal Sarcoma. Curr Oncol. 2023;30(5):4618-31. doi: https://doi.org/10.3390/curroncol30050349
13. Constantinidou A, Jones RL. Systemic therapy in retroperitoneal sarcoma management. J Surg Oncol. 2018;117(1):87-92. doi: https://doi.org/10.1002/jso.2493310.1002
14. Gopikrishna V. A report on case reports. J Conserv Dent. 2010;13(4):265-71. doi: https://doi.org/10.4103/0972-0707.73375
Recebido em 27/2/2024
Aprovado em 1/4/2024
Scientific-Editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.