ORIGINAL ARTICLE
Factors Associated with Neuropathic Pain in Cancer Patients Admitted to a Palliative Care Unit
Fatores Associados à Dor Neuropática em Pacientes com Câncer Admitidos em uma Unidade de Cuidados Paliativos
Factores Asociados al Dolor Neuropático en Pacientes con Cáncer Ingresados en una Unidad de Cuidados Paliativos
https://doi.org/10.32635/2176-9745.RBC.2024v70n2.4603
Patricia Almeida Chelles1; Livia Costa de Oliveira2; Luciana Silva Couto3; Simone Garruth dos Santos Machado Sampaio4; Anke Bergmann5
1-4Instituto Nacional de Câncer (INCA), Unidade de Cuidados Paliativos. Rio de Janeiro (RJ), Brasil. E-mails: patalchelles@gmail.com; lillycostaoliveira@gmail.com; lucianacouto.fisio@gmail.com; simonegarruth@gmail.com. Orcid iD: https://orcid.org/0000-0001-5687-0302; Orcid iD: https://orcid.org/0000-0002-5052-1846; Orcid iD: https://orcid.org/0009-0006-2870-567X; Orcid iD: https://orcid.org/0000-0001-5537-7399;
5INCA, Grupo de Pesquisa em Epidemiologia Clínica. Rio de Janeiro (RJ), Brasil. E-mail: abergmann@inca.gov.br. Orcid iD: https://orcid.org/0000-0002-1972-8777
Corresponding author: Patricia Almeida Chelles. INCA/HC IV/Unidade de Cuidados. Rua Visconde de Santa Isabel, 274 – Vila Isabel. Rio de Janeiro (RJ), Brasil. CEP 20560-120. E-mail: patalchelles@gmail.com
ABSTRACT
Introduction: Recognizing factors associated with pain in advanced cancer patients may favor a better multidisciplinary approach. Objective: To identify factors associated with the most prevalent type of pain in cancer patients admitted to a palliative care unit. Method: Cohort-study-based cross-sectional analysis of baseline data collected from cancer inpatients at the palliative care unit assisted by the physiotherapy service. Sociodemographic, clinical data and pain characteristics were obtained and analyzed according to the most prevalent type of pain at hospital admission. An odds ratio (OR) logistic regression was utilized as a measure of effect at a 95% confidence interval (95% CI). Results: A total of 62 patients were assessed, mostly women (69.3%) with less than 60 years old (67.7%), the most frequent tumor sites were gynecological (25.8%) and breast (16.1%). Local disease progression associated with metastasis was observed in 87.1% of all patients, with 50.0% presenting bone metastasis and 37.1%, a bone event. The most prevalent type was moderate to severe (69.3%) neuropathic pain (51.6%), associated with the presence of a bone event (OR = 3.16; 95% CI: 1.01-9.90) and less than 60 years old (OR = 4.08; 95% CI: 1.91 -17.52). Conclusion: Neuropathic pain was the most prevalent pain in cancer patients receiving palliative care associated with the presence of bone events and less than 60 years old.
Key words: Cancer Pain; Hospitalization; Neoplasm Metastasis; Palliative Care.
RESUMO
Introdução: Reconhecer os fatores associados à dor em pacientes com câncer avançado pode favorecer uma melhor abordagem multidisciplinar. Objetivo: Identificar os fatores associados ao tipo de dor mais prevalente em pacientes oncológicos internados em uma unidade de cuidados paliativos. Método: Análise transversal baseada em estudo de coorte de dados basais coletados de pacientes oncológicos internados na unidade de cuidados paliativos atendidos pelo serviço de fisioterapia. Dados sociodemográficos, clínicos e características da dor foram obtidos e analisados de acordo com o tipo de dor mais prevalente na admissão hospitalar. Uma regressão logística empregando o odds ratio (OR) foi utilizada como medida de efeito em um intervalo de confiança de 95% (IC 95%). Resultados: Foram avaliados 62 pacientes, a maioria do sexo feminino (69,3%), com idade inferior a 60 anos (67,7%), e os tumores mais frequentes foram os ginecológicos (25,8%) e mamários (16,1%). A progressão local da doença associada à metástase foi observada em 87,1% de todos os pacientes, 50,0% apresentaram metástase óssea e 37,1%, evento ósseo. O tipo de dor mais prevalente foi a neuropática (51,6%), moderada a intensa (69,3%). A dor neuropática foi associada à presença de evento ósseo (OR = 3,16; IC 95%: 1,01-9,90) e idade inferior a 60 anos (OR = 4,08; IC 95%: 1,91-17,52). Conclusão: A dor neuropática foi a mais prevalente em pacientes oncológicos em cuidados paliativos associada à presença de eventos ósseos e idade inferior a 60 anos.
Palavras-chave: Dor do Câncer; Hospitalização; Metástase Neoplásica; Cuidados paliativos.
RESUMEN
Introducción: Reconocer los factores asociados al dolor en pacientes con cáncer avanzado puede favorecer un mejor abordaje multidisciplinario. Objetivo: Identificar los factores asociados al tipo de dolor más prevalente en pacientes oncológicos ingresados en una unidad de cuidados paliativos. Método: Análisis transversal basado en un estudio de cohorte de datos basales recopilados de pacientes oncológicos ingresados en la unidad de cuidados paliativos atendidos por el servicio de fisioterapia. Datos sociodemográficos, clínicos y características del dolor según el tipo de dolor más prevalente al ingreso hospitalario. Se utilizó una regresión logística empleando el odds ratio (OR) como medida del efecto en un intervalo de confianza del 95% (IC del 95%). Resultados: Se evaluaron 62 pacientes, la mayoría del sexo femenino (69,3%), menores de 60 años (67,7%), y los tumores más frecuentes fueron ginecológicos (25,8%) y de mama (16,1%). Se observó progresión local de la enfermedad asociada con metástasis en el 87,1% de todos los pacientes, el 50,0% tuvo metástasis óseas y el 37,1% tuvo un evento óseo. El tipo de dolor más prevalente fue el neuropático (51,6%), moderado a severo (69,3%). El dolor neuropático se asoció con la presencia de evento óseo (OR = 3,16; IC 95%: 1,01-9,90) y edad menor de 60 años (OR = 4,08; IC 95%: 1,91-17,52). Conclusión: El dolor neuropático fue el dolor más prevalente en pacientes oncológicos sometidos a cuidados paliativos asociado a la presencia de eventos óseos y edad menor de 60 años.
Palabras clave: Dolor en Cáncer; Hospitalización; Metástasis de la Neoplasia; Cuidados Paliativos.
INTRODUCTION
Cancer pain can be caused by tumors, secondary to antineoplastic therapy or associated with other events not related to cancer, as immobility, pressure injury, muscle weakness or comorbidities, among others1-3. Its prevalence in advanced cancer, metastatic disease or terminal cancer is 54.6%4 and, according to Sampaio, Motta and Caldas2, about 93.0% of patients receiving palliative care may suffer intense pain during hospitalization.
Among the different types of pain, neuropathic pain results from injury to nerves or abnormal nerve function at any point along the neuronal pathway, from peripheral tissues to the central nervous system. It is relatively common, can be caused by surgical, chemotherapy and radiotherapy treatment, or by tumor invasion, and, when associated with oncological treatment, is usually moderate to intense1,5,6.
Bone metastases are common complications and one of the main nociceptive somatic pain causes in cancer patients. They frequently occur in the vertebrae, pelvic bones, long bones, and skull and can evolve with bone events, defined as the presence of one or more of the following clinical situations: pathological fracture, hypercalcemia associated with malignancy, spinal cord compression syndrome (SCCS), surgery to correct fractures and/ or deformities and radiotherapy to control severe pain. Bone events such as pathological fractures with compression and damage to nervous system structures (spinal cord, nerve roots, plexuses, or peripheral nerves) may occur in advanced stages, with extensive bone destruction7.
Cancer pain in general is a multifactorial and multidimensional symptom that includes physical, emotional, social, and spiritual aspects, a concept proposed by Cicely Saunders as “total pain”. Thus, its management should consider what pain causes in patients and how it affects their lives8,9. A thorough assessment is required to understand pain symptoms and their impacts, its cause, location, characteristics, type, intensity and behavior should be identified and treated whenever possible with both pharmacological and non-pharmacological procedures9,10.
Although several articles have been published and studies carried out on the subject, cancer pain is a frequent cause of hospitalization2 and remains a challenging symptom for healthcare professionals. In this context, building scientific evidence around pain-associated factors in advanced cancer patients can comprise an important strategy in care planning and in applying appropriate therapies for this particular group. Given that promoting pain relief and other symptoms to improve quality of life are guiding principles of palliative care8,9 the present study aims to identify factors associated with the most prevalent type of pain of cancer inpatients followed up by a physiotherapy service in a Brazilian reference palliative care unit.
METHOD
Cohort-study based cross-sectional analysis of baseline data collected from inpatients with advanced cancer from June 2021 to April 2022 and evaluated by the physiotherapy service of “Hospital do Cancer IV (HCIV)”, a palliative care unit of the Brazilian National Cancer Institute (INCA) in the city of Rio de Janeiro, RJ, Brazil. This is an excerpt from a broader project approved by INCA’s Institutional Review Board (IRB), CAAE (submission for ethical review) number. 46226921.7.0000.5274, report 4.729.007 on May 24, 2021 in compliance with Directive 466/201211 of the National Health Council. The guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) were followed12.
The sample size was calculated considering a death frequency of 90% among patients with advanced cancer and with more intense pain during hospital admission and a frequency of 60% in patients with less intense pain, with an alpha error of 5% and power of 80%, eventually reaching 62 patients enrolled in the study. A pilot study was previously carried out using the same proposed methodology to test the instruments designed for data collection. Modifications were suggested and implemented in the questionnaires and logistics have been improved. To ensure the quality of the data collected, skilled palliative care investigators participated of the study.
Both male and female patients aged 18 years or older with pain symptoms managed by INCA’s physiotherapy service according to the institutional routine at the time of admission (excluding epigastric pain, headache, dysuria, others), with a Karnofsky Performance Status (KPS) of over 30% who agreed to participate and signed the Informed Consent Form (ICF) were included. Patients disoriented/altered level of consciousness, with dyspnea, nausea/vomiting, bleeding or unable to understand the questions were excluded.
Data were collected during a face-to-face interview with the patient and review of the physical and electronic medical records. All the interviews were carried out within 72 hours from hospitalization. The following socioeconomic and demographic variables were obtained from medical records: age, sex, self-reported skin color, marital status, education and care network characteristics as shown in Table 1.
The clinical data collected were: primary tumor site, disease progression [local, regional locus (in the primary tumor + lymph node area) + distant metastasis], number of metastases (<2, >2), bone metastasis, bone events [hypercalcemia, pathological fracture and SCCS], previous cancer treatment (surgery, chemotherapy, radiotherapy or hormone therapy) and, comorbidities [systemic arterial hypertension (SAH), diabetes mellitus (DM) or others (heart disease, lung disease, others)].
The pain diagnostic criteria developed by the International Association for the Study of Pain (IASP) Special Interest Group on Neuropathic Pain (NeuPSIG) was applied to assess pain; these criteria classify neuropathic pain according to neurological injury history and plausible neuroanatomically pain distribution, if the pain is associated with sensory signals in the innervation territory of the nervous structure and diagnostic confirmation of injury or disease that explains neuropathic pain13.
Somatic nociceptive, visceral nociceptive, neuropathic and mixed pain types were considered, where data on symptom duration in days (<15, >15 days), pain intensity described by a visual numerical scale (VNS) obtained by requesting the patients to assign a score from 0 to 10 for their pain, with “0” being no pain and “10” being the most intense pain possible, were employed.
Pain was classified from zero (0) as no pain, one to three as mild or low intensity pain, four to six as moderate pain and seven to ten as severe or intense pain10,14, whose main location was [spine (cervical, thoracic and lumbar) and others (upper limbs, lower limbs, pelvis, thorax, abdomen, others)], spontaneous pain [yes or no (incidental or due to failure at the end of the administered drug dose)], localized pain [yes or no (radiated or referred)], improvement and worsening factors (lying down, changing positions, sitting, walking, deep breathing and others).
All the variables were analyzed according to the most prevalent type of pain, neuropathic pain [yes or no (somatic nociceptive, visceral nociceptive or mixed)] considering the study outcome.
The SPSS15 software (Statistical Package for the Social Sciences, Chicago, IL, USA) version 20.0 was utilized for the statistical analyses. Categorical variables were described as absolute and relative frequencies. Variables with missing data were described in the results notes. Pearson's chi-square test was used to compare age, sex, tumor type and type of pain between among and excluded patients. Crude and adjusted logistic regression models were performed to identify the factors associated with the most prevalent type of pain applying the odds ratio (OR) and 95% confidence interval (95% CI) as effect measures. Variables with a p < 0.20 in the crude analysis were included in the adjusted model. The final model was applied using the stepwise forward method designed to reduce confounding effects. Values were considered statistically significant when p <0.05.
RESULTS
A total of 296 patients felt pain upon admission during the study period. Of these, 62 were followed up by physiotherapy and were included herein. No statistically significant differences among included and excluded patients regarding age (p = 0.178), sex (p = 0.245), tumor type (p = 0.523) and type of pain (p = 0.112) were noticed.
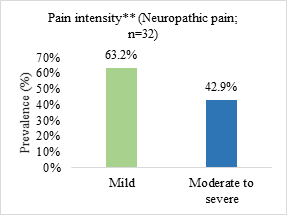
Neuropathic pain was the most prevalent (51.6%) and the most reported pain intensity was moderate to severe (69.3%) (Figure 1).
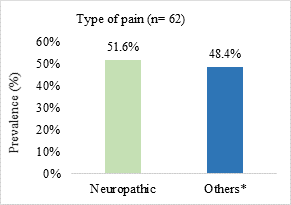
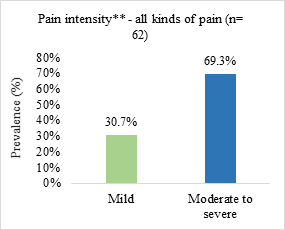
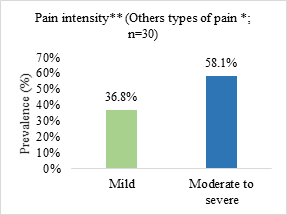
Figure 1. Prevalence of pain type and symptom intensity in patients admitted to a palliative care unit in Brazil (n=62)
n = number of observations.
*somatic nociceptive pain (n = 17; 27.4%)/visceral nociceptive pain (n = 8; 12.9%)/mixed pain (n = 5; 8.1%); **pain intensity assessed from the visual numerical scale (VNS) and classified as mild or weak (VNS from 1 to 3), moderate (VNS from 4 to 6) and strong or severe (VNS from 7 to 10).
Most patients were women (69.3%), less than 60 years old (67.7%), non-white (59.7%), with low education level (59.9%) and a present and fully participatory care network (70.3%) (Table 1).
Table 1. Sociodemographic profile of cancer patients admitted to a palliative care unit in Brazil and associations with neuropathic pain (n=62)
|
Variables |
Total n (%) |
Neuropathic pain n (%) |
Univariate regression |
|||
|
Yes 32 (51.6) |
Noa 30 (48.4) |
OR (CI 95%) |
pf |
|||
|
Age (years) |
|
|
|
|
|
|
|
> 60 |
20 (32.3) |
7 (21.9) |
13 (43.3) |
1.00 |
0.075 |
|
|
< 60 |
42 (67.7) |
25 (78.1) |
17 (56.7) |
2.73 (0.90-8.26) |
|
|
|
Sex |
|
|
|
|
|
|
|
Male |
19 (30.7) |
7 (21.9) |
12 (40.0) |
1.00 |
0.126 |
|
|
Female |
43 (69.3) |
25 (78.1) |
18 (60.0) |
2.38 (0.78-7.24) |
|
|
|
Skin color |
|
|
|
|
|
|
|
White |
25 (40.3) |
10 (31.2) |
15 (50.0) |
1.00 |
0.135 |
|
|
Non-whiteb |
37 (59.7) |
22 (68.8) |
15 (50.0) |
2.20 (0.78-6.19) |
|
|
|
Marital status |
|
|
|
|
|
|
|
With spouse |
33 (54.1) |
13 (41.9) |
20 (66.7) |
1.00 |
0.055 |
|
|
Without spousec |
28 (45.9) |
18 (58.1) |
10 (33.3) |
2.77 (0.98-7.85) |
|
|
|
Family income (MW)e |
|
|
|
|
|
|
|
>1 |
27 (48.2) |
13 (43.3) |
14 (53.9) |
1.00 |
0.433 |
|
|
<1 |
29 (51.8) |
17 (56.7) |
12 (46.1) |
1.52 (0.53-4.39) |
|
|
|
Education e |
|
|
|
|
|
|
|
<7 years |
33 (59.9) |
14 (46.7) |
19 (65.5) |
1.00 |
0.147 |
|
|
>7 years |
26 (44.1) |
16 (53.3) |
10 (34.5) |
2.17 (0.76-6.20) |
|
|
|
Care network characteristicse |
|
|
|
|
|
|
|
Present and fully participative |
38 (70.3) |
23 (85.2) |
15 (55.6) |
1.00 |
0.022 |
|
|
Otherd |
16 (29.7) |
4 (14.8) |
12 (44.4) |
4.60 (1.25-16.97) |
|
|
Captions: n = absolute frequency; % = relative frequency; OR = odds ratio; CI = confidence interval; MW = minimum wage.
Note: asomatic nociceptive pain (n= 17; 27.4%)/visceral nociceptive pain (n= 8; 12.9%)/mixed pain (n= 5; 8.1%); bnot white= black/brown/yellow; cno partner=divorced/widowed/single; dcare network absent/network present and partial participation; evariable with missing data; fp refers to univariate logistic regression; values in bold are statistically significant.
The most prevalent tumor site was gynecological (25.8%), followed by breast (16.1%), lung (9.7%) and gastrointestinal tract (GIT) (9.7%). Local disease progression associated with metastasis was observed in 87.1% of the investigated cases, with 50.0% presenting bone metastasis and 37.1% presenting a bone event. Chemotherapy (75.4%) was the previous cancer treatment mostly applied (Table 2).
Table 2. Clinical profile of cancer patients admitted to a palliative care unit in Brazil and association with neuropathic pain (n=62)
|
Variables |
Total n (%) |
Neuropathic pain
|
Univariate regression |
||
|
Yes 32 (51.6) |
Noa 30 (48.4) |
OR (CI 95%) |
ph |
||
|
Primary tumor site |
|
|
|
|
|
|
Breast |
10 (16.1) |
7 (21.9) |
3 (10.0) |
1.00 |
|
|
Gynecologicalb |
16 (25.8) |
7 (21.9) |
9 (30.0) |
0.33 (0.06-1.77) |
0.199 |
|
Lung |
6 (9.7) |
4 (12.5) |
2 (6.7) |
0.86 (0.10-7.51) |
0.889 |
|
HN |
3 (4.8) |
0 (0.0) |
3 (10.0) |
* |
|
|
CBT |
5 (8.1) |
4 (12.5) |
1 (3.3) |
1.71 (0.13-22.51) |
0.682 |
|
GITc |
6 (9.7) |
5 (15.6) |
1(3.3) |
2.14 (0.17-27.10) |
0.556 |
|
Prostate |
2 (3.2) |
0 (0.0) |
2 (6.7) |
* |
|
|
Othersd |
14 (22.6) |
5 (15.6) |
9 (30.0) |
0.24 (0.041-1.35) |
0.106 |
|
Disease progression |
|
|
|
|
|
|
Local |
8 (12.9) |
3 (9.4) |
5 (16.7) |
1.00 |
0.398 |
|
Regional site + distant metastasis |
54 (87.1) |
29 (90.6) |
25 (83.3) |
1.93 (0.42-8.91) |
|
|
Number of metastases |
|
|
|
|
|
|
< 2 |
35 (56.4) |
17 (48.6) |
18 (51.4) |
1.00 |
0.586 |
|
> 2 |
27 (43.6) |
15 (55.6) |
12 (44.4) |
1.32 (0.48-3.63) |
|
|
Bone metastasis |
|
|
|
|
|
|
No |
31 (50.0) |
13 (40.6) |
18 (60.0) |
1.00 |
0.130 |
|
Yes |
31 (50.0) |
19 (59.4) |
12 (40.0) |
2.19 (0.79-6.05) |
|
|
Bone eventse |
|
|
|
|
|
|
No |
39 (62.9) |
16 (50.0) |
23 (76.7) |
1.00 |
0.030 |
|
Yes |
23 (37.1) |
16 (50.0) |
7 (23.3) |
1.93 (1.02-3.79) |
|
|
Type of prior treatmentf |
|
|
|
|
|
|
Surgery |
|
|
|
|
|
|
No |
25 (41.0) |
12 (37.5) |
13 (44.8) |
1.00 |
0.562 |
|
Yes |
36 (59.0) |
20 (62.5) |
16 (55.2) |
1.35 (0.49-3.77) |
|
|
Chemotherapy |
|
|
|
|
|
|
No |
15 (24.6) |
6 (18.7) |
9 (31.0) |
1.00 |
0.266 |
|
Yes |
46 (75.4) |
26 (81.3) |
20 (69.0) |
1.95 (0.59-6.38) |
|
|
Radiotherapy |
|
|
|
|
|
|
No |
20 (32.8) |
8 (25.0) |
12 (41.4) |
1.00 |
0.174 |
|
Yes |
41 (67.2) |
24 (75.0) |
17 (58.6) |
2.12 (0.71-6.29) |
|
|
Hormone therapy |
|
|
|
|
|
|
No |
53 (86.9) |
27 (84.4) |
26 (89.7) |
1.00 |
0.542 |
|
Yes |
8 (13.1) |
5 (15.6) |
3 (10.3) |
1.60 (0.35-7.41) |
|
|
Comorbidities |
|
|
|
|
|
|
Hypertension |
|
|
|
|
|
|
No |
51 (82.3) |
26 (81.2) |
25 (83.3) |
1.00 |
0.830 |
|
Yes |
11 (17.7) |
6 (18.8) |
5 (16.7) |
1.15 (0.31-4.27) |
|
|
Diabetes mellitus |
|
|
|
|
|
|
No |
57 (91.9) |
31 (96.9) |
26 (86.7) |
1.00 |
0.174 |
|
Yes |
5 (8.1) |
1 (3.1) |
4 (13.3) |
4.77 (0.50-45.36) |
|
|
Othersg |
|
|
|
|
|
|
No |
56 (90.3) |
28 (87.5) |
28 (93.3) |
1.00 |
0.444 |
|
|
6 (9.7) |
4 (12.5) |
2 (6.7) |
1.81 (0.34-11.82) |
|
Captions: n = absolute frequency; % = relative frequency; OR = odds ratio; CI = confidence interval; HN = head and neck; CBT = connective bone tissue; GIT = gastrointestinal tract.* - unable to calculate.
Note: asomatic nociceptive pain (n = 17; 27.4%)/visceral nociceptive pain (n = 8; 12.9%)/ mixed pain (n = 5; 8.1%); bcervix and ovary; cesophagus, stomach, intestine and rectum; d Central Nervous System, melanoma, non-melanoma, thyroid and others; e Of these, one patient had hypercalcemia (one with neuropathic pain), six had pathological fractures (four with neuropathic pain) and 21 presented spinal cord compression (15 with neuropathic pain); ffour patients were treatment- naïve and 58 underwent some type of previous treatment; gheart disease, lung disease, others; hp refers to univariate logistic regression; values in bold indicate statistical significance.
All patients with bone events presented spine bone metastasis, with 65.22% reporting the spine as the main pain location, while 34.78% presented pain in another location (data not shown).
Pain lasting more than 15 days was the most frequent (59.7%), with the spine as the most affected site (29.0%). Spontaneous pain was present in 51.6% of the cases and localized pain, in 56.4% (Table 3).
Table 3. Reported characteristics of cancer patients admitted to a palliative care unit and their association with neuropathic pain (n=62)
|
Variables |
Total n (%) |
Neuropathic pain
|
Univariate regression |
||
|
Yes 32 (51.6) |
Noa 30 (48.4) |
OR (CI 95%) |
ph |
||
|
Duration (days) |
|
|
|
|
|
|
<15 |
25 (40.3) |
12 (37.5) |
13 (43.3) |
1.00 |
0.6401 |
|
>15 |
37 (59.7) |
20 (62.5) |
17 (56.7) |
1.27 (0.46-3.52) |
|
|
Pain location |
|
|
|
|
|
|
Spineb |
18 (29.0) |
12 (37.5) |
6 (20.0) |
1.00 |
0.134 |
|
Othersc |
44 (71.0) |
20 (62.5) |
24 (80.0) |
0.42 (0.13-1.31) |
|
|
Spontaneous |
|
|
|
|
|
|
Yes |
32 (51.6) |
14 (43.7) |
18 (60.0) |
1.00 |
0.203 |
|
Nod |
30 (48.4) |
18 (56.3) |
12 (40.0) |
1.93 (0.70-5.3) |
|
|
Localized |
|
|
|
|
|
|
Yes |
35 (56.4) |
9 (28.1) |
26 (86.7) |
1.00 |
<0.001 |
|
Noe |
27 (43.5) |
23 (71.9) |
4 (13.3) |
16.61 (4.50-61.23) |
|
|
Worsening factore |
|
|
|
|
|
|
To sit |
22 (47.8) |
11 (42.3) |
11 (55.0) |
1.00 |
0.394 |
|
Othersf |
24 (52.2) |
15 (57.7) |
9 (45.0) |
1.67 (0.51-5.40) |
|
|
Improvement factor |
|
|
|
|
|
|
Lying down |
8 (17.4) |
3 (11.5) |
5(25.0) |
1.00 |
0.242 |
|
Othersg |
38 (82.6) |
23 (88.5) |
15 (75.0) |
2.55 (0.53-12.31) |
|
Captions: n= absolute frequency; %= relative frequency; OR= odds ratio; CI= confidence interval; VNS = visual numerical scale.
Note: asomatic nociceptive pain (n=17;27.4%)/visceral nociceptive pain (n=8; 12.9%)/mixed pain (n=5; 8.1%); bcervical/thoracic/lumbar spine; cupper limbs/lower limbs/pelvis/thorax/abdomen/others; dincidental/failure at the end of dose; irradiated or referred; epatients chose more than one option; fChanging position/walking/staying down/breathing deeply; gChange of position/others; hp refers to univariate logistic regression; values in bold indicate statistical significance.
According to the crude analysis, the variables selected for the adjusted model were age, sex, self-reported skin color, marital status, education, care network characteristic, bone metastasis, bone events, radiotherapy as previous treatment, diabetes mellitus, pain location, and localized pain (Tables 1 to 3).
The adjusted model revealed that patients presenting bone events (OR=3.16; 95% CI: 1.01-9.90) and less than 60 years old (OR= 4.08; 95% CI: 1.91-17.52) were associated with neuropathic pain (Table 4).
Table 4. Final model of factors associated with neuropathic pain in cancer patients admitted to a palliative care unit in Brazil (n=62)
|
Variables |
Multivariate regression |
|
|
OR (CI 95%) |
pa |
|
|
Age (years) |
|
|
|
>60 |
1.00 |
0.030 |
|
<60 |
4.08 (1.91-17.52) |
|
|
Bone event |
|
|
|
No |
1.00 |
0.048 |
|
Yes |
3.16 (1.01-9.90) |
|
Captions: OR = odds ratio; CI = confidence interval.
Note: ap refers to multivariate logistic regression; values in bold indicate statistical significance.
DISCUSSION
Neuropathic pain was present in 51.6% of patients admitted to oncological palliative care in the present study, evaluated according to the IASP pain diagnostic criteria. Satija et al.16 observed a similar prevalence in their sample, where 54% of cancer patients undergoing palliative care at the outpatient clinics of three hospitals in India were identified with symptoms assessed with Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) pain scale self-reporting version.
Other studies, however, reached different findings. For example, a systematic review of prospective studies revealed that nearly one third of cancer patients experience neuropathic pain, ranging from 30.0% in oncology wards to 32.4% in oncology palliative care units17. In another assessment, Yanaizumi et al.18 reported a 30.6% prevalence of neuropathic pain among terminal cancer inpatients in Japan when applying the IASP diagnostic criteria, with colon/rectum (13%), lung (12%) and stomach (12%) as the most frequent tumor types. Belayneh et al.19 adopted the same diagnostic criteria and reported 24.9% of neuropathic pain in advanced cancer patients in Canadian palliative care centers, with gastrointestinal (26%), respiratory system (21%) and female genital organs (8%) as the most prevalent tumor sites. Finally, Mendes, Machado and Linartevichi5 reported 33% of neuropathic pain cases in their study by applying the painDETECT, a simple questionnaire based on neuropathic clinical symptoms to predict the likelihood of this type of pain in people with low back pain.
This type of pain is difficult to diagnose, which can lead to underdiagnosis16, justifying the differences of prevalence found in many studies. The aforementioned studies had different designs, applied other tools to identify neuropathic pain, in diverse scenarios and populations for another types of cancer.
Age is associated with higher rates of occurrence and other complications. Some authors correlate age with complaints of pain and suggest that older inpatients tend to report less severe pain than younger patients, who most likely report their suffering and e need for pain treatments20,21. In this regard, Oosterling et al.22 investigated an outpatient cancer population with a mean age of 57 years and reported the presence of neuropathic pain in about one in five patients. In another study, Hansen et al.23 observed that for cancer patients in palliative care, problems as pain, insomnia, nausea and emotional function tend to be less significantly severe with advanced age.
Belayneh et al.19 reported that being younger than 60 years old was a predictor of neuropathic pain in cancer patients undergoing palliative care, and that these patients present 1.9-fold significantly greater risk of neuropathic pain (p = 0.001). In another investigation, Sumimoto et al.24 observed that younger age was an independent clinical factor associated with the use of high-dose opioids (p = 0.001), which may be associated with intractable cancer pain in patients undergoing palliative care. These findings corroborate the results reported that being younger than 60 years of age was significantly associated with neuropathic pain.
Bone metastases and bone events are mechanisms that comprise the causes of neuropathic pain in patients with cancer25-27. The bone event prevalence reported herein was similar to that described by other authors and was significantly associated with neuropathic pain. One study verified a bone event prevalence of 45.1% in patients with bone metastasis27, while another reported a 34.7% rate28. Furthermore, Von Moos et al.28 observed that patients with bone events were at higher risk of increased pain and the use of opioid analgesics.
All patients presenting a bone event in the present study exhibited spine bone metastasis, which may be associated with the presence of neuropathic pain in view of the physiological mechanisms compatible with nerve root involvement in this region. Similarly, Faria et al.29 also described an association between a bone event, specifically SCCS, and vertebral pain, present in 81.1% of patients, most of whom with breast cancer, (46.7%), genitourinary tumors (27.8%) and lung cancer (11.1%). These types of tumor were more frequent in patients with neuropathic pain, and, according to the literature, bone metastasis in this type of tumors is frequent25.
The strength of this study is that the findings reported herein can help the development of better care strategies for advanced cancer patients, as early diagnosis of bone metastases even with nociceptive somatic pain, favoring adequate treatment and prevention of complications. In addition, the research site is a national reference unit in oncological palliative care in Brazil and its professionals are experienced in caring for this population.
Some limitations, however, may make it difficult to generalize the results. For example, only patients followed up by the physiotherapy service were included, which may have introduced a selection bias, as not all the patients in pain were evaluated. Furthermore, the study was carried out in a single institution that serves a population assisted by the Brazilian National Health System (SUS) with specific sociodemographic and clinical characteristics: only inpatients with pain were included, but outpatients and home care patients were not.
CONCLUSION
Neuropathic pain was the most prevalent type of pain in advanced cancer patients admitted to a palliative care unit, associated with patients younger than 60 years old and bone events. These findings may contribute to define care strategies for this population, guide future studies and contribute to expand the current knowledge.
Future multicenter studies with bigger samples and patients in different settings as outpatient units and in home care should be conducted.
CONTRIBUTIONS
All authors contributed to the study design. Patricia Almeida Chelles and Luciana Silva Couto prepared and collected the data. Patricia Almeida Chelles, Simone Garruth dos Santos Machado Sampaio, Anke Bergmann and Lívia Costa de Oliveira drafted and critically reviewed the manuscript; and all the authors reviewed the preliminary versions of the manuscript. All the authors approved the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
The author Anke Bergmann declares a potential conflict of interests due to her being the scientific editor of INCA’s Revista Brasileira de Cancerologia. The other authors do not have any conflict of interests.
FUNDING SOURCES
None.
REFERENCES
1. Sampaio LR, Moura CV, Resende MA. Physiotherapeutic resources in the treatment of oncological pain: literature review. Rev Bras Cancerol. 2005;51(4):339-46. doi: https://doi.org/10.32635/2176-9745.RBC.2005v51n4.1940
2. Sampaio SGSM, Motta LB, Caldas CP. Medication and pain control: experience of a brazilian palliative care referral center. Rev Bras Cancerol. 2019;65(2):e-13365. doi: https://doi.org/10.32635/2176-9745.RBC.2019v65n2.365
3. George B, Minello C, Allano G, et al. Opioids in cancer-related pain: current situation and outlook. Support Care Cancer. 2019;27(8):3105-18. doi: https://doi.org/10.1007/s00520-019-04828-8
4. Snijders RAH, Brom L, Theunissen M, et al. Update on prevalence of pain in patients with cancer 2022: a systematic literature review and meta-analysis. Cancers (Basel). 2023;15(3):591. doi: https://doi.org/10.3390/cancers15030591
5. Mendes CMC, Machado DM, Linartevichi VF. Neuropathic index in oncological patients and pharmacological conduct. FJH. 2020;2(4):424-8. doi: https://doi.org/10.35984/fjh.v2i4.264
6. Couceiro TCM, Lima LC, Coutinho Júnior MP, et al. Prevalence of neuropathic pain in patients with cancer. Br J Pain. 2018;1(3):231-5. doi: https://doi.org/10.5935/2595-0118.20180045
7. Zajączkowska R, Kocot-Kępska M, Leppert W, et al. Bone pain in cancer patients: mechanisms and current treatment. Int J Mol Sci. 2019;20(23):6047. doi: https://doi.org/10.3390/ijms20236047
8. Associação Brasileira de Fisioterapia em Oncologia. Manual de Condutas e Práticas Fisioterapêuticas em Cuidados Paliativos Oncológicos da ABFO. Rio de Janeiro: Thiene Revinter Publicações; 2021.
9. Academia Nacional de Cuidados Paliativos. Manual de Cuidados Paliativos da ANCP. 3. ed. Rio de Janeiro: Atheneu; 2021.
10. Instituto Nacional De Câncer José Alencar Gomes da Silva. Cuidados paliativos: vivências e aplicações práticas do Hospital do Câncer IV [Internet]. Rio de Janeiro: INCA, 2021. [acesso 2023 jun 15]. Disponível em: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//cuidados_paliativos-hciv.pdf
11. Conselho Nacional de Saúde (BR). Resolução n° 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília, DF. 2013 jun 13; Seção I:59.
12. Malta MS, Cardoso LO, Bastos FIPM, et al. Iniciativa STROBE: subsídios para a comunicação de estudos observacionais. Rev Saúde Pública. 2010;44(3):559-65.
13. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599-1606. doi: https://doi.org/10.1097/j.pain.0000000000000492
14. Fortunato JGS, Furtado MDS, Hirabae LFDA, et al. "Scales of pain in the critically ill patient: an integrative review". Rev HUPE. 2013 [acesso 2023 jun 15];12(3):110. Disponível em: https://link.gale.com/apps/doc/A372554603/AONE?u=anon~e1184ef5&sid=googleScholar&xid=67c55fee
15. SPSS®: Statistical Package for Social Science (SPSS) [Internet]. Versão 20.0. [Nova York]. International Business Machines Corporation. [acesso 2024 jan 9]. Disponível em: https://www.ibm.com/br-pt/spss?utm_content=SRCWW&p1=Search&p4=43700077515785492&p5=p&gclid=CjwKCAjwgZCoBhBnEiwAz35Rwiltb7s14pOSLocnooMOQh9qAL59IHVc9WP4ixhNTVMjenRp3-aEgxoCubsQAvD_BwE&gclsrc=aw.ds
16. Satija A, Joad AK, Rana SPS, et al. The burden of cancer-related neuropathic pain: a multi-centric cross-sectional observational study from North India. Indian J Palliat Care. 2021;27(1):104-08. doi: https://doi.org/10.4103/ijpc.ijpc_277_20
17. Roberto A, Deandrea S, Greco MT, et al. prevalence of neuropathic pain in cancer patients: pooled estimates from a systematic review of published literature and results from a survey conducted in 50 italian palliative care centers. J Pain Symptom Manage. 2016;51(6):1091-02.e4. doi: https://doi.org/10.1016/j.jpainsymman.2015.12.336
18. Yanaizumi R, Nagamine Y, Harada S, et al. Prevalence of neuropathic pain in terminally ill patients with cancer admitted to a general ward: a prospective observational study. J Int Med Res. 2021;49(1):300060520987726. doi: https://doi.org/10.1177/0300060520987726
19. Belayneh M, Fainsinger R, Nekolaichuk C, et al. Edmonton classification system for cancer pain: comparison of pain classification features and pain intensity across diverse palliative care settings in canada. J Palliat Med. 2023;26(3):366-75. doi: https://doi.org/10.1089/jpm.2022.0187
20. Yoon SL, Scarton L, Duckworth L, et al. Pain, symptom distress, and pain barriers by age among patients with cancer receiving hospice care: comparison of baseline data. J Geriatr Oncol. 2021;12(7):1068-75. doi: https://doi.org/10.1016/j.jgo.2021.04.008
21. Olden AM, Holloway R, Ladwig S, et al. Palliative care needs and symptom patterns of hospitalized elders referred for consultation. J Pain Symptom Manage. 2011;42(3):410-8. doi: https://doi.org/10.1016/j.jpainsymman.2010.12.005
22. Oosterling A, te Boveldt N, Verhagen C, et al. Neuropathic pain components in patients with cancer: prevalence, treatment, and interference with daily activities. Pain Pract. 2016;16(4):413-21. doi: https://doi.org/10.1111/papr.12291
23. Hansen MB, Ross L, Petersen MA, et al. Age, cancer site and gender associations with symptoms and problems in specialised palliative care: a large, nationwide, register-based study. BMJ Support Palliat Care. 2022;12(e2):e201-e210. doi: https://doi.org/10.1136/bmjspcare-2019-001880
24. Sumimoto H, Hayashi K, Kimura Y, et al. Factors associated with cancer-related pain requiring high-dose opioid use in palliative cancer patients. Palliat Med Rep. 2021;2(1):237-41. doi: https://doi.org/10.1089/pmr.2021.0037
25. Tsukamoto S, Kido A, Tanaka Y, et al. Current overview of treatment for metastatic bone disease. Curr Oncol. 2021;28(5):3347-72. doi: https://doi.org/10.3390/curroncol28050290
26. Silva GT, Bergmann A, Thuler LCS. Incidence and risk factors for bone metastasis in non-small cell lung cancer. Asian Pac J Cancer Prev. 2019;20(1):45-51. doi: https://doi.org/10.31557/apjcp.2019.20.1.45
27. Hong S, Youk T, Lee SJ, et al. Bone metastasis and skeletal-related events in patients with solid cancer: a Korean nationwide health insurance database study. PLoS One. 2020;15(7):e0234927. doi: https://doi.org/10.1371/journal.pone.0234927
28. von Moos R, Body JJ, Egerdie B, et al. Pain and analgesic use associated with skeletal-related events in patients with advanced cancer and bone metastases. Support Care Cancer. 2016;24(3):1327-37. doi: https://doi.org/10.1007/s00520-015-2908-1
29. Faria EM, Araujo BP, Chelles PA, et al. Prognostic factors and functionality in metastatic spinal cord compression: cohort study. Rev Bras Cancerol. 2022;68(2):e-182160. doi: https://doi.org/10.32635/2176-9745.RBC.2022v68n2.2160
Recebido em 5/3/2024
Aprovado em 16/5/2024
Executive-editor: Letícia Casado. Orcid iD: https://orcid.org/0000-0001-5962-8765
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.