ORIGINAL ARTICLE
Translation, Cross-Cultural Adaptation, Reliability and Construct Validity of the FACT-EGFRI-18 Quality of Life Instrument from English into Portuguese
Tradução, Adaptação Transcultural, Confiabilidade e Validade de Construto do Instrumento de Qualidade de Vida FACT-EGFRI-18 do Inglês para o Português
Traducción, Adaptación Transcultural, Confiabilidad y Validez de Constructo del Instrumento de Calidad de Vida FACT-EGFRI-18 del Inglés al Portugués
https://doi.org/10.32635/2176-9745.RBC.2024v70n3.4672
Luiza Erthal de Britto Pereira Kassuga Roisman1; Anke Bergmann2; Luiz Claudio Santos Thuler3
1Instituto Nacional de Câncer (INCA), Hospital do Câncer I. Rio de Janeiro (RJ), Brasil. E-mail: draluizakassuga@gmail.com. Orcid iD: https://orcid.org/0000-0001-8403-7764
2,3INCA, Programa de Epidemiologia Clínica. Rio de Janeiro (RJ), Brasil. E-mail: abergmann@inca.gov.br; lthuler@gmail.com. Orcid iD: https://orcid.org/0000-0002-1972-8777; Orcid iD: https://orcid.org/0000-0003-2550-6537
Corresponding author: Anke Bergmann. Rua André Cavalcanti, 37 - sala 9 (anexo) – Centro. Rio de Janeiro (RJ), Brasil. CEP 20231-050. E-mail: abergmann@inca.gov.br
ABSTRACT
Introduction: Adverse epidermal growth factor receptor (EGFR) dermatological events affect many patients and can impair the patient's quality of life (QoL), leading to dose reduction or discontinuation of therapy. Objective: To carry out the translation, cross-cultural adaptation, reliability and validity of the Functional Evaluation of Cancer Therapy - Epidermal Growth Factor Receptor Inhibitor 18 (FACT-EGFRI-18) to be utilized in Portuguese. Method: Study to evaluate the cross-cultural adaptation, reliability and construct validation of the FACT-EGFRI-18 translated into Portuguese. To evaluate cross-cultural adaptation, semantic analysis was performed by ten patients and content analysis by experts. Reliability was assessed by internal consistency analysis using Cronbach's alpha coefficient. Construct validity was determined by the correlation between the translated FACT-EGFRI-18 and the Dermatology Life Quality Index (DLQI) answered by 30 participants with cancer undergoing treatment with EGFR. A descriptive analysis of the study population was performed and Cronbach's alpha coefficient was calculated to determine the internal consistency of the Portuguese version of the FACT-EGFRI-18 and Pearson's demonstration coefficient was applied to determine the transparency between the questionnaires. ANOVA test was performed to compare the mean FACT-EGFRI-18 score according to demographic variables. Results: The semantic evaluation showed that all the participants understood the items of the translated questionnaire, which revealed strong internal consistency (Cronbach's alpha = 0.89) and validity (Pearson’s correlation = 0.66). Conclusion: The FACT-EGFRI-18 was easily understood by the patients and the results support its reliability and validity.
Key words: ErbB Receptors; Translating; Reproducibility of Results; Surveys and Questionnaires; Quality of Life.
RESUMO
Introdução: Eventos dermatológicos adversos do receptor do fator de crescimento epidérmico (EGFR) afetam muitos pacientes e podem prejudicar a sua qualidade de vida (QV), levando à redução da dose ou à descontinuação da terapia. Objetivo: Realizar a tradução, adaptação transcultural, confiabilidade e validade do Functional Evaluation of Cancer Therapy - Epidermal Growth Factor Receptor Inhibitor 18 (FACT-EGFRI-18) para uso em português. Método: Estudo para avaliar a adaptação transcultural, a fidedignidade e a validação de construto do FACT-EGFRI-18 traduzido para o português. Para avaliar a adaptação transcultural, foram realizadas a análise semântica por dez pacientes e a análise do conteúdo por especialistas. A fidedignidade foi avaliada pela análise de consistência interna utilizando o coeficiente alfa de Cronbach. A validade do construto foi determinada pela correlação entre o FACT-EGFRI-18 traduzido e o Índice de Qualidade de Vida em Dermatologia (DLQI), respondidos por 30 participantes com câncer em tratamento com EGFR. Foi realizada a análise descritiva da população do estudo, o coeficiente alfa de Cronbach foi calculado para determinar a consistência interna da versão em português do FACT-EGFRI-18, e o coeficiente de correlação de Pearson aplicado para determinar a correlação entre os questionários. O teste ANOVA foi realizado para comparar a média do escore FACT-EGFRI-18 de acordo com as variáveis demográficas. Resultados: A avaliação semântica mostrou que houve compreensão dos itens do questionário traduzido por todos os participantes; o questionário apresentou forte consistência interna (alfa de Cronbach = 0,89) e validade (correlação de Pearson = 0,66). Conclusão: O FACT-EGFRI-18 demonstrou ser facilmente compreendido pelos pacientes e os resultados apoiam a sua confiabilidade e validade.
Palavras-chave: Receptores ErbB; Tradução; Reprodutibilidade dos Testes; Inquéritos e Questionários; Qualidade de Vida.
RESUMEN
Introducción: Los eventos dermatológicos adversos del receptor del factor de crecimiento epidérmico (EGFR) afectan a muchos pacientes y pueden afectar la su calidad de vida (CdV), lo que lleva a una reducción de la dosis o la interrupción del tratamiento. Objetivo: Realizar la traducción, adaptación transcultural, confiabilidad y validez de la Functional Evaluation of Cancer Therapy - Epidermal Growth Factor Receptor Inhibitor 18 (FACT-EGFRI-18) para su uso en portugués. Método: Estudio para evaluar la adaptación transcultural, confiabilidad y validación de constructo del FACT-EGFRI-18 traducido al portugués. Para evaluar la adaptación transcultural, el análisis semántico fue realizado por diez pacientes y el análisis de contenido por expertos. La confiabilidad se evaluó mediante análisis de consistencia interna utilizando el coeficiente alfa de Cronbach. La validez de constructo se determinó mediante la correlación entre el FACT-EGFRI-18 traducido y el Índice de Calidad de Vida en Dermatología (DLQI) respondido por 30 participantes con cáncer en tratamiento con EGFR. Se realizó el análisis descriptivo de la población del estudio y se calculó el coeficiente alfa de Cronbach para determinar la consistencia interna de la versión traducida de FACT-EGFRI-18 y el coeficiente de correlación de Pearson se aplicó para determinar la correlación entre los cuestionarios. Se realizó la prueba ANOVA para comparar la puntuación media FACT-EGFRI-18 según variables demográficas. Resultados: La evaluación semántica mostró que todos los participantes entendieron los ítems traducidos del cuestionario; el cuestionario mostró una fuerte consistencia interna (alfa de Cronbach = 0,89) y validez (correlación de Pearson = 0,66). Conclusión: Los pacientes entendieron fácilmente el FACT-EGFRI-18 y los resultados respaldan su confiabilidad y validez.
Palabras clave: Receptores ErbB; Traducción; Reproducibilidad de los Resultados; Encuestas y Cuestionarios; Calidad de Vida.
INTRODUCTION
Adverse dermatological events of epidermal growth factor receptor (EGFR) affect 71% to 90% of patients whose frequency is variable during the course of the therapy1-3. The most common toxicity is papulopustular eruption, but nail changes (paronychia and granuloma), xeroderma, capillary alterations (changes in color and texture, alopecia, hypertrichosis, trichomegaly and trichiasis), fissures in the hands and feet, pruritus, mucositis, telangiectasias, hyperpigmentation and nasal vestibulitis are too3-12. No studies on the incidence of dermal reactions utilizing EGFR were found for the Brazilian population.
EGFRI cutaneous reactions can impair the patient’s quality of life (QoL), leading to dose decreases or therapy discontinuation13-15. Currently, the Functional Assessment of Cancer Therapy – Epidermal Growth Factor Receptor Inhibitor 18 (FACT-EGFRI-18) is the only validated questionnaire for the specific assessment of the consequences of cutaneous EGFRI toxicity on health related QoL (HRQoL)16 and no translated and validated tool for Portuguese in this group of patients is available.
This study aims to translate and perform cross-cultural adaptation, reliability and construct validity of the FACT-EGFRI-18 quality of life instrument from English into Portuguese in patients undergoing epidermal growth factor receptor inhibitor therapy.
METHOD
Cross-sectional observational study of the Portuguese translation, cross-cultural adaptation of the original FACT-EGFRI-18 English questionnaire, and construct validation of the FACT-EGFRI-18 translated into Portuguese.
The FACT-EGFRI-18 is based on 18-questions Likert scale to evaluate the effect of adverse dermatological EGFRI reactions on patient’s HRQoL. It was originally developed and validated in English16 based on the Functional Assessment of Side-Effects to Therapy – EGFRI (FAST-EGFRI), the first questionnaire created for this purpose17. The FACT-EGFRI-18 is divided into three dimensions: physical, social/emotional and functional well-being. Its questions address skin, nail and hair side effects. The answers for each item range from zero to four (zero="not at all", one="a little", two="somewhat", three="a lot", four="very much") and the patient should take into consideration the week prior to the interview. The score ranges from zero to 72 and the result is calculated by means of a reverse score, where each item should subtract four from the response. The calculation of an item equal to zero indicates greater impact on QoL, while four means less impact. The result of these subtractions should then be multiplied by 18, and subsequently divided by the number of answered items to obtain the final score. Thus, participants with the highest symptom severity receive a lower overall score.
The standard translation methodology adopted by the Functional Assessment of Chronic Illness Therapy (FACIT) organization was applied herein18,19. Initially, the FACT-EGFRI-18 was translated from English to Portuguese by two independent health professionals (T1 and T2), fluent in both languages, one of them residing in Brazil and the other in the USA. The authors of the study reconciled these two versions, solved the discrepancies and generated a single questionnaire in Portuguese (reconciled version – RCV).
This reconciled version was then translated back into English by a sworn translator, creating the retrotranslated version (RTV). The RTV version was submitted to FACIT professionals for evaluation who, after comparing with the original questionnaire in English, suggested modifications, considering semantic equivalence concepts, referential meaning of the terms and words utilized. After these changes, a fourth version was written in Portuguese and revised by a Portuguese health professional to adapt the questionnaire language to other Portuguese-speaking countries.
FACIT determined that ten patients using EGFRI, five Brazilians and five Portuguese residents in Portugal should answer the final Portuguese FACT-EGFRI-18 version. The patients were recruited in September 2015 through a request to the Oncology Service to refer patients using EGFRI to the Dermatology Service or via the hospital pharmacy, which provided a list of patients using EGFRI. The inclusion criterion was the use of EGFRI and the exclusion criterion was the presence of previous dermatological diseases affecting more than 30% of the body surface.
After completing the questionnaire, the translation was tested through cognitive debriefing, which is a standardized interview to inform the patients whether the items are relevant to them, both personally and culturally. The patient interview form in Portuguese was sent by FACIT and contained some demographic information, general questions about the translated questionnaire and specific questions on each one of the 18 items.
For the construct validity, 30 patients with colorectal or non-small cell lung cancer treated with cetuximab, erlotinib or gefitinib were selected and answered three questionnaires: 1) the FACT-EGFRI-18 translated into Portuguese, 2) the Dermatology Life Quality Index (DLQI), translated and validated into Portuguese, and 3) a sociodemographic questionnaire.
A descriptive analysis of the study population was carried out by means of central tendency measures for the quantitative variables and absolute and relative frequency for the qualitative variables. Cronbach's alpha coefficient was calculated to determine the internal consistency of the FACT-EGFRI-18 in Portuguese and the DLQI, interpreted as: <0.4, weak; 0.4 to 0.74, moderate; 0.75 to 0.9, strong; and >0.9, very strong20. Pearson’s correlation coefficient was applied to determine the correlation between both questionnaires, which was defined as: 0.00-0.19, very weak; 0.20-0.39, weak; 0.40-0.59, moderate; 0.60-0.79, strong; and 0.80-1.00, very strong21. The ANOVA test was performed to compare the mean FACT-EGFRI-18 score between genders (male versus female), age ranges (< 65 years versus ≥ 65 years), therapy target type (cetuximab versus erlotinib versus gefitinib) and adverse effects (presence versus absence) presented by the patients. It was also used to compare the mean FACT-EGFRI-18 score in patients with papulopustular facial eruptions and those without facial lesions. p < 0.05 was considered as statistically significant. Data were analyzed using the IBM SPSS version 23.022.
INCA’s Research Ethics Committee (CEP) approved the study, report 1223364, CAAE (submission for ethical review), number 48093415.9.0000.5274, in compliance with Directive 466/201223 of the National Health Council. All participants were informed about the objectives of this study and signed the Informed Consent Form (ICF). The translation of the FACT-EGFRI-18 was authorized by the FACIT. The use of the DLQI was authorized by Professor Andrew Finlay.
RESULTS
FACT-EGFRI-18 transcultural translation and adaptation
Two translators, three conciliators (authors of this study), a sworn translator, FACIT professionals, a reviewer from Portugal and an expert bilingual Portuguese participated of the transcultural translation and adaptation of the FACT-EGFRI-18. Throughout the process, some terms were modified to facilitate their comprehension, adjusting cultural differences between the English and Portuguese languages and preserving the meaning of the original questionnaire (Supplementary table 1).
For example, the item “My skin or scalp feels irritated” (ST4) was translated by T1 as “Sinto minha pele ou couro cabeludo irritados" and by T2 as “Minha pele ou couro cabeludo ficam irritados", in Portuguese. The reconciliation opted for "My skin or scalp gets irritated," which was then back-translated as "My skin or scalp gets irritated." FACIT professionals evaluated that the verb "gets" rather than "feels" could be a reference for the past or future, not conveying the concept that the patient feels that their skin or scalp is irritated when they answered the questionnaire.
Additional reviews by FACIT professionals and a bilingual Portuguese expert confirmed that the final Portuguese version is an adequate translation of the English questionnaire.
Pre-test of the FACT-EGFRI-18
The main author of this study and a FACIT representative in Portugal participated in the pre-test. Of the five Brazilian patients, two (40.0%) were males and three (60.0%) were females, all of them (100%) had colorectal cancer.
Question ten of the patient interview form asked the participant to indicate which statement best described his/her current level of activity. Four patients answered "Some symptoms, but I do not need to stay in bed during the day while I am awake" and one (20.0%) answered "I need to stay in bed less than 50% of the day while I am awake".
All the Brazilians patients interviewed (n=5) in the pre-test understood the questionnaire's questions, did not have any difficulty to understand the terminology or language, or found any irrelevant or offensive items. For instance, item ST32 asked about "the condition of my skin", and the patients responded "general condition that differs from normal", "very irritated", "irritated skin", "skin with lesions" and "injury, dryness, sensitivity of my skin", demonstrating full comprehension of what was being asked.
They denied there was anything else about their disease that should have been included, but the fourth interviewee responded question 16 ("Now I will ask some questions about item ST7: "My skin bleeds easily" Explain the meaning of this item in your own words.") that "Nose vessels become more sensitive and when the skin becomes irritated, they bleed easier," revealing the involvement of the nasal cavity. This symptom is not addressed in FACT-EGFRI-18.
Two patients (40.0%), at the end of the interview, reported having another comment about the questionnaire. The first evaluated the Portuguese translation as "It is well done and very clear" and the fifth added that "My ears are scratching and my nose is injured", also referring to other adverse effects that were not addressed by FACT-EGFRI-18. The three (60.0%) other participants only reported that the questionnaire is easy to understand, with no additional comments.
The results of the five Brazilian cases were sent electronically (scanned questionnaires and patient’s interview form) to the FACIT group, which combined them with data from the five Portuguese cases, and chose the version utilized in the pre-test as the final Portuguese version of FACT-EGFRI-18 (Figure 1).
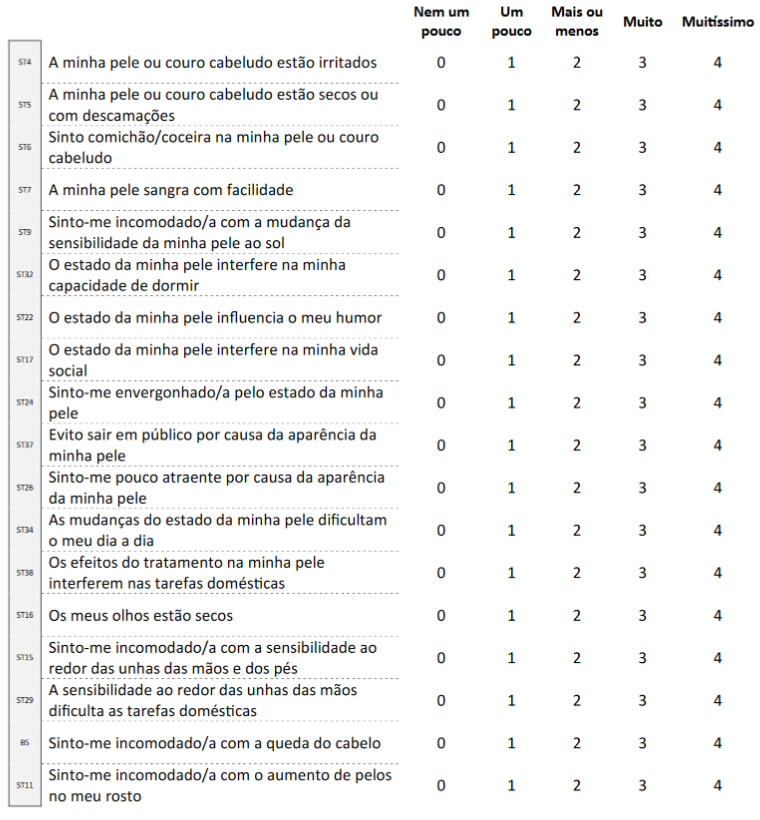
Figure 1. Final Portuguese version of FACT-EGFRI-18
Construct validation
For the construct validation, 30 patients have been included. 17 (56.7%) patients were males and 13 (43.3%) were females, in the age range of 34 to 81 years old and mean age of 61.5 ± 10.7. A predominance of colorectal cancer (66.7%) was observed and cetuximab was the most commonly target therapy applied (66.7%) (Table 1).
Table 1. Sociodemographic information of the participants (n = 30) of the construct validation of the final Portuguese version of FACT-EGFRI-18
|
Variables |
n (%) |
|
Age (years) |
|
|
34-57 58-65 66-81 |
8 (26.7) 12 (40.0) 10 (33.3) |
|
Gender |
|
|
Male Female |
17 (56.7) 13 (43.3) |
|
Fitzpatrick phototyping scale |
|
|
2 3 4 5 6 No information |
3 (10.0) 7 (23.3) 6 (20.0) 7 (23.3) 6 (16.7) 2 (6.7) |
|
Education |
|
|
Incomplete/complete elementary school Incomplete/complete high school Incomplete/complete university Post-graduation |
10 (33.3) 9 (30.0) 10 (33.3) 1 (3.3) |
|
Marital status |
|
|
Married Divorced Widow/Widower Single |
19 (63.3) 5 (16.7) 3 (10.0) 3 (10.0) |
|
Primary site |
|
|
Colorectal Lung |
20 (66.7) 10 (33.3) |
|
Staging |
|
|
2 3 4 |
1 (3.3) 7 (23.3) 22 (73.3) |
|
Target therapy |
|
|
Cetuximab Erlotinib Gefitinib |
20 (66.7) 2 (6.7) 8 (26.7) |
93.3% of the patients reported one or more mucocutaneous alterations as adverse dermatological effects, the most common was papulopustular eruption in 76.7%, while 26.7% developed a grade three eruption (Table 2).
Table 2. Side effects reported by participants (n = 30) from the construct validation of the definitive Portuguese version of FACT-EGFRI-18
|
Side effects |
Grade 1 |
Grade 2 |
Grade 3 |
Total |
|
Classified by CTCAE version 4.3 |
|
|
|
|
|
Papulo pustular eruption |
7 (23.3) |
8 (26.7) |
8 (26.7) |
23 (76.7) |
|
Xerosis |
1(3.3) |
7 (23.3) |
11(36.7) |
19 (63.3) |
|
Pruritus |
14(46.7) |
4 (13.3) |
0(0.0) |
18 (60.0) |
|
Alopecia |
8 (26.7) |
1 (3.3) |
0 (0.0) |
9 (30.0) |
|
Paronychia |
4 (13.3) |
3 (10.0) |
0 (0.0) |
7 (23.3) |
|
Hypertrichosis |
6 (20.0) |
0 (0) |
0 (0) |
6 (20.0) |
|
Mucositis |
1 (3.3) |
5 (16.7) |
0 (0) |
6 (20.0) |
|
Photosensitivity |
3 (10.0) |
2 (6.7) |
0 (0) |
5 (16.7) |
|
Telangiectasia |
3 (10.0) |
0 (0) |
0 (0) |
3 (10.0) |
|
Hyperpigmentation |
1 (3.3) |
0 (0) |
0 (0) |
1 (3.3) |
|
Not classified by CTCAE version 4.3 |
|
|
|
|
|
Peri ungual pyogenic granuloma-like |
|
|
|
3 (10.0) |
|
Erythema |
|
|
|
9 (30.0) |
|
Scalp flaking |
|
|
|
6 (20.0) |
|
Others |
|
|
|
5 (16.7) |
|
Acral fissures |
|
|
|
2 (6.7) |
|
Blepharitis |
|
|
|
1 (3.3) |
|
Nasal vestibulitis |
|
|
|
1 (3.3) |
|
Liquenification |
|
|
|
1 (3.3) |
The mean scores of the FACT-EGFRI-18 in Portuguese and the DLQI are listed in Supplementary tables 2, 3 and 4. Pearson's correlation coefficient was 0.66, indicating a strong correlation between both questionnaires. This is an inverse correlation, since the FACT-EGFRI-18 score is reverse, whereas the DLQI score is not. Cronbach's alpha coefficient of the Portuguese-language version of the FACT-EGFRI-18 was 89%, showing a strong internal consistency, while the DLQI presented a Cronbach's alpha coefficient of 66%, revealing moderate internal consistency.
When comparing the FACT-EGFRI-18 scores in patients with and without adverse dermatological effects, the mean score was lower in the presence of cutaneous reactions (except for hair shaft alterations and hyperpigmentation). However, xerosis (p = 0.027) and telangiectasias (p = 0.005) were the only adverse effects presenting a statistically significant difference (Table 3).
Table 3. Mean FACT-EGFRI-18 scores of patients (n = 30) of construct validation according to the presence or absence of dermatological effects
|
Side effect |
Presence Mean (SD) |
Absence Mean (SD) |
p value |
|
Papulo pustular eruption |
56.4 (11.5) |
62.9 (12.1) |
0.204 |
|
Xerosis |
54.3 (11.9) |
64.0 (8.9) |
0.027 |
|
Pruritus |
56.4 (11.4) |
60.0 (12.5) |
0.426 |
|
Alopecia |
54.8 (10.8) |
59.2 (12.1) |
0.354 |
|
Paronychia |
56.0 (12.7) |
58.4 (11.7) |
0.639 |
|
Periungualpyogenic granuloma-like |
51.7 (1.2) |
58.6 (12.2) |
0.344 |
|
Hypertrichosis |
55.8 (11.8) |
58.4 (11.9) |
0.643 |
|
Hair shaft alterations |
59.4 (7.8) |
57.6 (12.5) |
0.755 |
|
Mucositis |
51.3 (12.8) |
59.5 (11.1) |
0.130 |
|
Photosensitivity |
51.8 (10.9) |
59.1 (11.7) |
0.211 |
|
Telangiectasia |
40.7 (9.1) |
59.8 (10.5) |
0.005 |
|
Hyperpigmentation |
59.0 (-) |
57.8 (11.9) |
0.924 |
|
Erythema |
56.6 (9.7) |
58.4 (12.7) |
0.696 |
|
Scalpflaking |
57.2 (11.6) |
58.0 (12.0) |
0.874 |
|
Caption: SD = standard deviation. |
|||
No statistically significant difference regarding sex, age and type of target therapy was observed in the mean scores of the FACT-EGFRI-18 (Table 4).
Table 4. Mean FACT-EGFRI-18 scores of participants (n = 30) for construct validation according to sex, age and target therapy
|
Variables |
Mean (SD) |
p value |
|
Sex |
|
|
|
Male |
61.4 (8.8) |
0.057 |
|
Female |
53.2 (13.8) |
|
|
Age (years) |
|
|
|
< 65 |
58.6 (12.0) |
0.685 |
|
≥ 65 |
56. 8 (11.8) |
|
|
Target therapy |
|
|
|
Cetuximab |
58.4 (10.1) |
0.192 |
|
Erlotinib |
43.5 (13.4) |
|
|
Gefitinib |
60.1 (14.1) |
|
|
SD = standard deviation. |
||
DISCUSSION
This study conducted the translation, cross-cultural adaptation and validation of the FACT-EGFRI-18 to be utilized in Portuguese. This was the second translation of the original English FACT-EGFRI-18. The first was translated into Dutch by Boers-Doets et al. applying the FACIT methodology17-19.
In another study published by Boers-Doets14, six Dutch patients suggested questions about cutaneous side effects not addressed in FACT-EGFRI-18, such as sensitive eyes, bleeding and pimples in the nasal cavity, dry mouth, tingling sensation, and scalp pain. Only one Brazilian patient during the pre-test suggested questions about other treatment reactions, such as ear itching and nasal wounds, and a second participant, who, although did not offer any additional comments, answered question 16 of the interview form as "Nose vessels become more sensitive and when the skin becomes irritated, bleeds easier," demonstrating involvement of the nasal cavity. The other patients reported only that the questionnaire is easy to understand, with no further observations.
Clabbers et al.24 evaluated the QoL of 85 Dutch patients who presented adverse dermatological EGFRI effects during the first six weeks of treatment using four questionnaires. The means of the FACT-EGFRI-18 scores calculated in each of the seven weeks (0, 1, 2, 3, 4, 5 e 6) were 69.5 (SD 5.23), 67.0 (SD 8.03), 63.8 (SD 7.00), 64.6 (SD 6.95), 62.6 (SD 9.75), 63.2 (SD 8.97) and 61.6 (SD 10.1), respectively.
In the validation of the FACT-EGFRI-18 in Portuguese the mean score was 57.9 (± 11.7), lower than the seven means reported by Clabbers et al.24 which indicates greater QoL impairment of the individuals analyzed . This can be attributed to the difficulty of access to health services and the lower socioeconomic status of the Brazilian population, with potential repercussions on the understanding of guidelines on EGFRI reactions provided by health professionals and on the acquisition of cosmetics and drugs for their control.
The internal consistency of the FACT-EGFRI-18 in Portuguese was strong (Cronbach's alpha=89%) for the patients evaluated, while internal DLQI consistency was 66% (moderate internal consistency). This is due to the fact that DLQI is a more general tool for assessing the QoL of individuals presenting dermatological alterations, while the FACT-EGFRI-18 is specific to the effects of EGFRI treatment, possibly influencing the results encountered.
The QoL items that evaluated the physical symptoms had a greater impact, as demonstrated by other authors13,14,24. Papulopustular eruptions were also the most prevalent adverse effect (76.7%), corroborating the literature1,2,11.
While the mean FACT-EGFRI-18 scores were lower in the presence of almost all adverse effects (except hair shaft changes and hyperpigmentation after papulopustular eruption), these differences were statistically significant only for xerosis and telangiectasia (p < 0.05). Clabbers et al. found that xerosis and pruritus are the most damaging effects on patient’s QoL24. Therefore, it is paramount to advise patients on possible side effects, including specific guidelines for dry skin and the consequences of papulopustular eruption.
Although no statistical significance was found between the FACT-EGFRI-18 scores of patients with facial papulopustular eruption when compared to those without these lesions, its clinical importance must be highlighted, since lesions in this area are more visible and difficult to camouflage. More apparent papules and pustules may eventually raise other people's questions about the patient's illness and treatment, leading to lower self-esteem and impacting the QoL.
A comparison was carried out between the means of the FACT-EGFRI-18 total scores for each patient according to sex, age and type of target therapy. Although not statistically significant, women presented lower scores than men. The likely explanation for this finding is that female patients are more sensitive to adverse skin effects, which have a greater impact on appearance and care about herself. In the study carried out by Clabbers et al. no differences between sex or type of cancer were observed, but patients younger than 50 years old presented higher mean score (p = 0.015) in the functional domain. Patients older than 81 years presented greater impact on the physical domain (p = 0.028) compared to patients with mean age ranging from 61 to 70 years old24.
The limitation of this study was the relatively small sample. However, the choice of five Brazilian participants and five Portuguese patients for pre-test was guided by the FACIT. The ideal number for validation would be ten times the number of items. However, at INCA, there is a limited quota for treatment with target therapy, not exceeding 40 to 50 patients. In addition, the study counted on the participation of patients presenting different types of cancer, the use of distinct types of EGFRI and dissimilar adverse dermatological effects. Furthermore, some side effects are interdependent variables, which in addition to the small number of participants, may have contributed to the decrease of statistical significance when comparing the Portuguese version of the FACT-EGFRI-18 scores with the variables analyzed herein.
CONCLUSION
The FACT-EGFRI-18 in Portuguese has been validated and can be used to measure the QoL of patients undergoing EGFRI treatment in Brazil. The existence of a more specific tool to measure the impact on the QoL can help researchers and clinicians to better evaluate these patients, allowing improved approach to adverse dermatological effects and, consequently, the adoption of measures to increase treatment adherence.
CONTRIBUTIONS
Luiza Erthal de Britto Pereira Kassuga Roisman contributed to the study design, acquisition, analysis and interpretation of the data and drafting of the manuscript. Anke Bergmann and Luiz Claudio Santos Thuler contributed to the study design, analysis and interpretation of the data and critical review. All the authors approved the final version to be published.
DECLARATION OF CONFLICT OF INTERESTS
The author Anke Bergmann declares a potential conflict of interests due to her being the scientific editor of INCA’s Revista Brasileira de Cancerologia. The other authors do not have any conflict of interests.
FUNDING SOURCES
Anke Bergmann and Luiz Claudio Santos Thuler are recipients of a productivity research scholarship of the National Council for Scientific and Technological Development (CNPq).
REFERENCES
1. Chiang TY, Hsu HC, Chern YJ, et al. Skin toxicity as a predictor of survival in metastatic colorectal cancer patients treated with anti-egfr: fact or fallacy? Cancers (Basel). 2023;15(6):1663. doi: https://doi.org/10.3390/cancers15061663
2. Lacouture ME, Mitchell EP, Piperdi B, et al. Skin Toxicity Evaluation Protocol With Panitumumab (STEPP), a phase ii, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J ClinOncol. 2010;28:1351-7.
3. Saito Y, Uchiyama K, Takekuma Y, et al. Risk factor analysis for anti-epidermal growth factor receptor monoclonal antibody-induced skin toxicities in real-world metastatic colorectal cancer treatment. Support Care Cancer. 2023;31(8):504. doi: https://doi.org/10.1007/s00520-023-07973-3
4. Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth fator receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-26.
5. Saito Y, Uchiyama K, Takekuma Y, et al. Evaluation of the additional prophylactic effect of topical steroid ointment to systemic minocycline against anti-epidermal growth factor antibody-induced skin toxicities in metastatic colorectal cancer treatment. Support Care Cancer. 2023;32(1):8. doi: https://doi.org/10.1007/s00520-023-08195-3
6. Deslandres M, Sibaud V, Chevreau C, et al. Cutaneous side effects associated with epidermal growth factor receptor and tyrosine kinase inhibitors. Ann Dermatol Venereol. 2008; (esp1):16-24. doi: https://doi.org/10.1016/s0151-9638(08)70093-0
7. Osio A, Mateus C, Soria JC, et al. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol. 2009;161:515-21.
8. Belum VR, Cercek A, Sanz-Motilva V, et al. Dermatologic adverse events to targeted therapies in lower GI cancers: clinical presentation and management. Curr Treat Options Oncol. 2013;14:389-404.
9. Sibaud V, Robert C. Pigmentary disorders induced by anticancer agents. Part II: targeted therapies. Ann Dermatol Venereol. 2013;140(4):266-73. doi: https://doi.org/10.1016/j.annder.2013.01.442
10. Ruiz JN, Belum VR, Boers-Doets CB, et al. Nasal vestibulitis due to targeted therapies in cancer patients. Support Care Cancer. 2015;23(8):2391-8. doi: https://doi.org/10.1007/s00520-014-2580-x
11. Lupu I, Voiculescu N, Bacalbasa N, et al. Cutaneous complications of molecular targeted therapy used in oncology. J Med Life. 2016;9(1):19-25.
12. Barton-Burke M, Ciccolini K, Mekas M, et al. Dermatologic reactions to targeted therapy: a focus on epidermal growth factor receptor inhibitors and nursing care. Nurs Clin North Am. 2017;52(1):83-113. doi: https://doi.org/10.1016%2Fj.cnur.2016.11.005
13. Wagner LI, Lacouture ME. Dermatologic toxicities associated with EGFR inhibitors: the clinical psychologis’s perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncology (Williston Park). 2007;21(Sup11):34-6.
14. Boers-Doets CB, Gelderblom H, Lacouture ME, et al. Experiences with the FACT-EGFRI-18 instrument in EGFRI-associated mucocutaneous adverse events. Support Care Cancer. 2013;21(7):1919-26. doi: https://doi.org/10.1007/s00520-013-1752-4
15. Rosen AC, Case EC, Dusza SW, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. AM J Clin Dermatol. 2013;14:327-33. doi: https://doi.org/10.1007/s40257-013-0021-0
16. Wagner LI, Berg SR, Gandhi M, et al. The development of a Functional Assessment of Cancer Therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18). Suppot Care Cancer. 2013;21(4):1033-41. doi: https://doi.org/10.1007/s00520-012-1623-4
17. Boers-Doets CB, Gelderblom H, Lacouture ME, et al. Translation and linguistic validation of the FACT-EGFRI-18 quality of life instrument from English into Dutch. Eur J Oncol Nurs. 2013;17(6):802-7. doi: https://doi.org/10.1016/j.ejon.2013.03.004
18. Bonomi AE, Cella DF, Hahn EA, et al. Multilingual translation of the Functional Assessment of Cancer Therapy (FACT) quality of life measurement system. Qual Life Res. 1996;5(3):309-20. doi: https://doi.org/10.1007/bf00433915
19. Eremenco SL, Cella D, Arnold BJ. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Eval Health Prof. 2005;28(2):212-32. doi: https://doi.org/10.1177/0163278705275342
20. Fleiss JL. The design and analysis of clinical experiments. New York: John Wiley & Sons Inc; 1986.
21. Evans JD. Straightforward statistics for the behavioral sciences. Pacific Grove: Brooks/Cole Publishing; 1996.
22. SPSS®: Statistical Package for Social Science (SPSS) [Internet]. Versão 23.0. [Nova York]. International Business Machines Corporation. [acesso 2023 mar 9]. Disponível em: https://www.ibm.com/br-pt/spss?utm_content=SRCWW&p1=Search&p4=43700077515785492&p5=p&gclid=CjwKCAjwgZCoBhBnEiwAz35Rwiltb7s14pOSLocnooMOQh9qAL59IHVc9WP4ixhNTVMjenRp3-aEgxoCubsQAvD_BwE&gclsrc=aw.ds
23. Conselho Nacional de Saúde (BR). Resolução n° 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União, Brasília, DF. 2013 jun 13; Seção I:59.
24. Clabbers JM, Boers-Doets CB, Gelderblom H, et al. Xerosis and pruritus as major EGFRI associated adverse events. Support Care Cancer. 2016;24:513-21. doi: https://doi.org/10.1007%2Fs00520-015-2781-y
Recebido em 10/4/2024
Aprovado em 18/6/2024
Executive-editor: Letícia Casado. Orcid iD: https://orcid.org/0000-0001-5962-8765
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.