LITERATURE REVIEW
Analgesic Potential of Flavonoids for Cancer-Associated Pain and its Treatments: Systematic Review
Potencial Analgésico dos Flavonoides na Dor Associada ao Câncer e ao seu Tratamento: Revisão Sistemática
Potencial Analgésico de los Flavonoides para el Dolor Asociado al Cáncer y sus Tratamientos: Revisión Sistemática
https://doi.org/10.32635/2176-9745.RBC.2025v71n2.4976
Maycon Giovani Santana¹; Rachel Verdan Dib²; Matheus Cavalcante de Deus3; Ana Lúcia Teodoro5; Denise Gonçalves Priolli6
1,3Oncologia D’OR. São Paulo (SP), Brasil. E-mail: g.belinasi@gmail.com; mathdedeus@gmail.com. Orcid iD: https://orcid.org/0000-0003-2084-668X; Orcid iD: https://orcid.org/0009-0002-2346-6885
²Instituto Nacional de Câncer (INCA). Rio de Janeiro (RJ), Brasil. E-mail: dibvrachel@gmail.com. Orcid iD: https://orcid.org/0000-0001-9684-1979
5A. C. Camargo Cancer Center. São Paulo (SP), Brasil. E-mail: a.teodoro2011@gmail.com. Orcid iD: https://orcid.org/0000-0002-3453-2908
6Faculdade de Ciências da Saúde Pitágoras de Codó. Codó (MA), Brasil. E-mail: depriolli@gmail.com. Orcid iD: https://orcid.org/0000-0003-3190-4013
Corresponding author: Maycon Giovani Santana, IDOR. Av. Brigadeiro Luís Antônio, 5001, 4º andar – Jardim Paulista. São Paulo (SP), Brasil. CEP 04502-001. E-mail: g.belinasi@gmail.com
ABSTRACT
Introduction: Pain is a prevalent and debilitating symptom in cancer patients, reducing quality of life. Its management is often challenging, with conventional pharmacological treatments associated with undesirable side effects. Natural compounds as flavonoids have garnered attention among emerging therapeutic approaches. Flavonoids are phenolic compounds abundant in the plant kingdom, recognized for their antitumor, anti-inflammatory, anti-allergic, and antiangiogenic properties. However, their analgesic potential is yet to be explored. Objective: Literature review of the analgesic potential of flavonoids in cancer pain and/or treatment-related. Method: A search was conducted at the PubMed, SciELO, Lilacs, and Cochrane Library databases with the keywords “cancer”, “flavonoids,” and “pain”. Inclusion criteria were clinical studies published between 2010 and 2024 in English, Portuguese, or Spanish, with patients over 18 years of age, that investigated the association among cancer, flavonoids, and pain. All the studies were submitted to risk of bias analysis. The study was approved by PROSPERO and based on PRISMA. Results: 14 clinical trials were included. The most frequently studied compound was Epigallocatechin gallate, for breast, lung, head and neck, and esophagus (n=9) cancers. All the studies investigated flavonoids in treatment-related pain, demonstrating pain reduction in cases of radiodermatitis, mucositis, esophagitis, and onycholysis. Flavonoid administration, either prophylactic or therapeutic, was well tolerated when applied topically or orally. Conclusion: Flavonoids exhibit promising analgesic potential in cancer-related pain, with minimal or no reported side effects. Further clinical studies are needed to elucidate their effectiveness in this context.
Key words: Neoplasms/drug therapy; Flavonoids/pharmacology; Pain Management.
RESUMO
Introdução: A dor é um sintoma prevalente e incapacitante em pacientes com câncer, contribuindo para redução da qualidade de vida. Seu manejo é desafiador, com tratamentos farmacológicos convencionais associados a efeitos colaterais indesejáveis. Entre as abordagens terapêuticas emergentes, compostos naturais como flavonoides têm recebido atenção. Flavonoides são compostos fenólicos abundantes no reino vegetal, que possuem propriedades antitumorais, anti-inflamatórias, antialérgicas e antiangiogênicas, embora seu potencial analgésico seja pouco explorado. Objetivo: Revisar a literatura acerca do potencial analgésico dos flavonoides na dor oncológica e/ou relacionada ao tratamento. Método: Foi realizada uma pesquisa nas bases PubMed, SciELO, Lilacs e Biblioteca Cochrane utilizando as palavras-chave "câncer", "flavonoides" e "dor". Foram incluídos estudos clínicos publicados entre 2010 e 2024, em inglês, português ou espanhol, com pacientes acima de 18 anos. Foram considerados estudos que avaliaram a relação entre câncer, flavonoides e dor. Todos os estudos foram submetidos à análise de risco de viés. O estudo foi aprovado pelo PROSPERO e elaborado a partir do PRISMA. Resultados: Foram incluídos 14 estudos clínicos. O composto mais frequentemente estudado foi a epigalocatequina galato, avaliado em cânceres de mama, pulmão, cabeça e pescoço e esófago (n=9). Todos os estudos investigaram flavonoides na dor relacionada ao tratamento, com redução álgica em radiodermatite, mucosite, esofagite e onicólise. A administração de flavonoides, profilática ou terapêutica, foi bem tolerada tanto por via tópica quanto oral. Conclusão: Os flavonoides demonstram potencial analgésico promissor na dor associada ao câncer, com poucos ou nenhum efeito colateral. Estudos adicionais são necessários para elucidar sua eficácia.
Palavras-chave: Neoplasias/tratamento farmacológico; Flavonoides/farmacologia; Manejo da Dor.
RESUMEN
Introducción: El dolor es un síntoma debilitante en pacientes con cáncer, reduciendo la calidad de vida. Su tratamiento es complicado, ya que los fármacos convencionales suelen causar efectos secundarios. Entre los enfoques emergentes, los flavonoides, compuestos fenólicos del reino vegetal, han recibido atención por sus propiedades antitumorales, antiinflamatorias y antiangiogénicas. No obstante, su potencial analgésico aún está poco explorado. Objetivo: Revisar la literatura sobre el potencial analgésico de los flavonoides en el dolor oncológico y el asociado a tratamientos. Método: Se realizó una búsqueda en PubMed, SciELO, Lilacs y Cochrane Library utilizando las palabras clave “cáncer”, “flavonoides” y “dolor”. Se incluyeron estudios clínicos publicados entre 2010 y 2024 en inglés, portugués o español, con pacientes mayores de 18 años. Se evaluaron estudios que investigaron la relación entre cáncer, flavonoides y dolor, y se realizó un análisis del riesgo de sesgo. El estudio fue aprobado por PROSPERO y se basó en PRISMA. Resultados: Se incluyeron 14 estudios clínicos. El galato de epigalocatequina galato fue el compuesto más estudiado, evaluado en cánceres de mama, pulmón, cabeza y cuello, y esófago (n=9). Todos los estudios investigaron el uso de flavonoides en el dolor relacionado con el tratamiento, observando una reducción en casos de radiodermitis, mucositis, esofagitis y onicólisis. La administración de flavonoides, profiláctica o terapéutica, fue bien tolerada tanto por vía tópica como oral. Conclusión: Los flavonoides muestran un prometedor potencial analgésico en el dolor asociado al cáncer, con efectos secundarios mínimos o inexistentes. Se requieren más estudios clínicos para confirmar su eficacia.
Palabras clave: Neoplasias/quimioterapia; Flavonoides/farmacología; Manejo del Dolor.
INTRODUCTION
During the cancer patient's journey, several events cause loss of performance. Among the lines of treatment, the main tool in cancer therapy is the association between surgical resection and radiotherapy and chemotherapeutic compounds, which may damage the healthy tissue. In this sense, local and systemic toxicity triggers side effects that are often limiting to the patient and with loss of quality of life1,2.
Pain is a common symptom of cancer and its treatment3. A study by Breivik et al. in 2009 evaluated 5,000 adults with cancer, showing that 56% of them suffered from moderate to severe pain at least monthly4. Cancer patients may develop hyperalgesia, allodynia, and spontaneous pain, impacting their quality of life5. However, pain can be relieved in up to 90% of these patients6 and, in despite of management efficacy, some studies show that pain remains poorly controlled for many of them6,7.
In the oncologic patient, pain can be related to the tumor invasion process to adjacent tissues, compressing peri-tumoral structures; for example, phantom pain associated with resection in surgical treatment, and peripheral neuropathy associated with chemotherapy or radiotherapy where there is tissue damage and pain, such as mucositis8,9 Furthermore, the patient's experience related to pain can positively or negatively influence the clinical outcome10.
The medications proposed to relieve pain are often associated with other side effects, as fatigue, sleep apnea, decreased libido, nausea, vomiting, and constipation11-14. Thus, it is necessary to search for new compounds with fewer side effects and similar efficacy to control pain.
Plants and their secondary metabolites have been used to treat various pathological conditions. In many regions with little access to modern medicine, plants are the primary source for home treatment of some diseases15,16. The science of natural products has discovered many substances used today.
Flavonoids, one of the main biocomposites, are the focus of tumor biology researches in recent decades. These are compounds of polyphenolic origin widely distributed in the plant kingdom and found in aglycones, glycosides, or methylated derivatives, usually detected in a healthy diet containing fruits and vegetables and concentrated in teas, wines, apples, onions, and cocoa17.
One of its most important mechanisms of actions is the antioxidant property, preventing damage caused by free radicals through direct neutralization of reactive oxygen species, activation of antioxidant enzymes, chelation of metals, reduction of α-tocopheryl radicals, inhibition of oxidases, reduction of oxidative stress caused by nitric oxide, increase in uric acid levels, and increase of the activity of low molecular weight antioxidants. The antioxidant capacity of some flavonoids, as Epigallocatechin gallate (EGCG), is higher than that of vitamins C and E18,19.
The molecular structure of flavonoids is based on the flavilium core, composed of three phenolic rings. Variations in the central phenolic ring give rise to flavonoid classes, as flavonols, flavones, catechins, flavanones, anthocyanidins, and isoflavonoids, and lead to the formation of compounds with distinct rates of absorption and bioavailability, responsible for different pharmacological actions of flavonoids in vivo19-21.
Flavonoids have a broad pharmacological activity already described, presenting antioxidant, anti-inflammatory, cardioprotective, vasorelaxant, antibacterial, antiviral, hepatoprotective, anti-proliferative, chemopreventive, neuroprotective, and antiangiogenic effects19,22-35. Although the literature points to the potential of flavonoids in pain management, little is known about their cancer-related analgesic potential. Thus, the objective of the present literature review is to evaluate the potential of flavonoids in the management of cancer-related pain and/or its treatment.
METHOD
Systematic review registered at PROSPERO36 (International Prospective Register of Systematic Reviews), number CRD42022311072, utilizing PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)37.
Relevant articles published in English, Portuguese and Spanish were searched at the databases PubMed, SciELO, Lilacs, and Cochrane Library from February 6, 2022 to September 25, 2024 applying the keywords combined with the Boolean operators AND OR for closed vocabularies: cancer AND pain OR cancer pain AND flavonoids OR flavonols according to DeCS/MESH (Descritores em Ciências da Saúde/Medical Subject Headings).
The authors selected the studies independently and, in case of any disagreement, a discussion was held to reach a consensus. The full text of the studies that met the inclusion criteria was separated for further evaluation. To expand the coverage of the literature, reference lists of the studies included and systematic reviews identified during the screening process were also investigated.
Studies associating cancer, flavonoids, and pain, utilizing control groups (flavonoid-treated versus conventional analgesic-treated or untreated), from 2010 to 2024, have been included, since there are few studies on the subject especially in the last five years addressing patients with confirmed diagnosis of pain and cancer, regardless of the tumor site or stage, aged 18 years or older (pain was associated with cancer or oncologic treatment (chemotherapy/radiotherapy/surgery). Studies investigating patients with diagnoses other than cancer, different types of symptoms, not addressing pain, off the scope, literature reviews and low level of scientific evidence were excluded.
Two authors collected the data and information (author's name, year of publication, method, sample type, and results) that met the inclusion criteria, and another author checked the information independently. The quality of the evidence and the risk of bias were assessed using the Cochrane Collaboration Risk of Bias Tool (RoB 2.0) for randomized clinical trials38, and Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I), for non-randomized studies39. Each study was categorized for risk of bias into low, high, and unclear. Any disagreements were discussed and resolved by another author.
Following the analysis, 15 studies collected at the Cochrane Database have been excluded due to other comorbidities, as hemorrhoids, postpartum pain, and osteoarthritis. Among the five pre-selected studies, four were excluded because they involved different compounds or were preclinical studies with animal models.
Similarly, 186 studies collected at PubMed database were excluded because they addressed unrelated pathologies, as cardiovascular diseases, arthritis, and other inflammatory processes, in addition to studies utilizing different compounds, flavonoids outside the analgesic context, or literature reviews/pre-clinical studies. As a result, after pre-selecting 50 studies, 37 articles have been excluded and 13 were included in the review.
Eventually, 13 articles have been identified at PubMed and one at the Cochrane Library (Figure 1), resulting in 14 studies summarized in Table 1. No results were found at SciELO and Lilacs.
All selected studies are in English, 50% of which were randomized (n=7). Of these, 71.42% were double-blinded (patient and investigator, n=5). 28.57% were single-blinded, only the patient (n=2). The studies investigated a minimum of 20 and a maximum of 180 patients.
Among the cancer topographies (Figure 2), 26.66% were lung cancer (n=4), 26.66%, breast cancer (n=4), 20%, prostate cancer (n=3), 13.33%, head and neck cancer (n=2), 6.66%, esophageal (n=1), and one (n=1), gynecological. One study evaluated two types of cancer (breast and prostate).
Epigallocatechin gallate was the most evaluated flavonoid by 62.28% of the studies (n=9), due to its efficacy in improving pain with few side effects associated with the compounds. The most common effects were burning and nausea40. In cases of topical application, there were no reports of adverse effects (Table 1).
The studies used the Common Terminology Criteria for Adverse Events (CTCAE)41-43, Radiation Therapy Oncology Group (RTOG)41,42,44, and European Organization for Research and Treatment of Cancer (EORTC)44 to assess toxicity. A questionnaire was used to assess the quality of life40,42,45,46. In addition, pain was also assessed using the Verbal Numeric Scale40,42,44,47,48. For skin toxicity, pain, burning, itching, pulling, and tenderness were assessed using the Skin Toxicity Assessment Tool in cases of patients undergoing radiotherapy41. Mucositis was assessed using a specific scale49.
In addition to radiotherapy, the chemotherapy drugs utilized were cisplatin, etoposide, fluorouracil, docetaxel, and cabazitaxel49,50.
The studies were statistically analyzed using the paired or unpaired t-test, chi-square, Mann-Whitney, and ANOVA. All statistical tests considered a significance level of less than or equal to 0.05.
The risk of bias was assessed individually for each selected study using Cochrane's assessment tool for randomized and non-randomized trials (RoB 2.0 and ROBINS-I, respectively) (Figure 3).
Each study was analyzed individually for methodological quality, according to questionnaires established by the Cochrane tools for systematic reviews. Seven randomized studies were assessed by Risk of Bias 2.0 for randomized studies. The main issue was bias due to missing results, because of loss to follow-up mainly associated with side effects in patients in the placebo group. The other seven studies were assessed using the ROBINS-I tool for non-randomized studies, with bias in the measurement of outcome and no-blinding of the participants.
DISCUSSION
Flavonoids, polyphenolic compounds of natural origin, are easily found in fruits and vegetables, and besides innumerable health benefits, their toxicity is low, emerging as new alternative therapies for some diseases as cancer. These molecules are known for their antioxidant effects and, therefore, for their direct action on oxidative stress. However, there is a wide range of benefits of flavonoids, as anti-inflammatory, antioxidant, antiallergic, antiproliferative, neuroprotective, antitumor, and antiangiogenic actions17,55,56.
Pain is the most reported and feared symptom for cancer patients. About 90% of patients experience pain at different stages of the disease progression. It is reported by 33% of survivors, 59% of those on treatment, 64% with active disease, and up to 80% in advanced stages and/or terminally ill, being the main cause for seeking medical care4,57.
The type of pain varies according to its origin. About 93% of patients complain of pain related to cancer itself, arising from bone lesions (spinal cord compression, skull metastases, fractures, and long bone lesions), nerve infiltration in epidural and meningeal sinuses, visceral infiltration in pleura, liver, peritoneum, and pancreas, and vascular infiltration, triggering stroke, thromboembolism, and vena cava obstruction. In addition, 21% of pain complaints are associated with treatment. The complaints depend on the modality of choice: surgery (post-thoracotomy, post-amputation), post-radiotherapy, post-chemotherapy (especially when submitted to platinum compounds, paclitaxel, vincristine, and bortezomib). There is also the pain complaint associated with mucositis, enteritis, myalgia, and arthralgia, as well as peripheral neuropathy58.
Pain management is based on the analgesic ladder postulated by the World Health Organization (WHO)59. In addition, the National Comprehensive Cancer Network has developed a guideline to systematize the care of patients with pain. The medications of choice vary between non-steroidal anti-inflammatory drugs (dipyrone), weak opioids (codeine), and strong opioids (morphine), further to the potential implementation of adjunctive medications as corticoids, antidepressants, anticonvulsants, and neuroleptics to help manage the symptoms1.
Although the guidelines help to treat pain associated with cancer or its treatment, many patients remain with uncontrolled pain. In addition, the prescribed medications are associated with other side effects, as fatigue, sleep apnea, decreased libido, nausea, vomiting, and constipation11-13. Thus, new studies that seek to support the treatment of cancer patients with the development of new drugs, reduction of pain complaints, and improvement of quality of life are essential.
Recent studies highlight flavonoids as antinociceptive agents in animal models for chronic pain, including pain associated with inflammation, neuropathic pain, and cancer pain60,61. Quercetin, for example, in a dose-dependent administration, significantly reduces thermal hyperalgesia and mechanical paclitaxel-induced allodynia62 or cold exposure63. Furthermore, an animal model of neuropathic pain induced by constriction injury showed that quercetin's analgesic effect was superior to gabapentin and morphine64.
Additionally, there are published studies demonstrating the action of flavonoids in delaying cognitive decline, reducing neuronal cell death, and improving memory related to neuroprotective effects, antioxidant properties, and anti-inflammatory action28,65. In addition, there are also reports of anxiolytic potential66,67 due to affinity and selectivity at benzodiazepine receptor sites and actions at GABA (gamma-aminobutyric acid) receptors 67. The mechanism of action of flavonoids involves the suppression of inflammation (via Tumor Necrosis Factor-α, Interleukin-12, Interferon-γ, Interferon-α, Interleukin-8, cyclooxygenase-2, and prostaglandin) and oxidative stress52,68, as well as the modulation of GABAergic69,70 and opioid pathways71,72.
The analysis of the tumor site of the patients investigated by the studies reveals alignment with global cancer incidence data. The studies focused on lung cancer, currently the most prevalent and lethal cancer worldwide, as well as breast and prostate cancers, are highly prevalent among women and men, respectively73.
Although other highly incident cancers as colorectal and gastric were not directly addressed, recent preclinical studies have investigated paclitaxel and oxaliplatin which are drugs commonly utilized in therapeutic protocols for these tumors. These agents are associated with painful complications, including peripheral neuropathy. The findings suggest that prophylactic or therapeutic administration of these drugs is associated with reduced chronic neuropathy, alleviation of allodynia, and behavioral alterations related to pain74-77.
Additionally, preclinical analyses also evaluated the efficacy of flavonoids in managing pain associated with bone invasion or metastasis, a condition commonly observed in breast, prostate, and lung cancers. The results are promising, with improvement in pain, pain-related behavior, reduction of inflammation, and inhibition of tumor necrosis factor (TNFα), a cytokine with proliferative activity, tumorigenesis, and cell differentiation69,71,78-80. Considering that about 93% of the patients have pain associated with cancer itself58, clinical studies evaluating the effect of these molecules, based on in vitro and in vivo results, should be conducted to promote alternatives for cancer pain management.
Injury to the nail bed in patients undergoing chemotherapy is common, causing disfigurement and pain. In 2018, a study evaluated the effectiveness of a balm containing herbal oils rich in bioactive polyphenolics in protecting nails through its reported anti-inflammatory, analgesic, antioxidant, and antimicrobial properties. The authors randomized 60 prostate and breast cancer patients to evaluate the topical application of the balm two to three times daily compared to oil control. The pain was assessed from the questionnaire application. Reported symptoms were significantly lower between the start and end of chemotherapy in the balm-treated group compared to the control group. In addition, the plant balm significantly reduced chemotherapy-related damage to the nail bed, improving quality of life46.
In 2010, Ahmad et al. conducted a pilot study with 27 patients diagnosed with prostate cancer undergoing radiation therapy, divided into a therapy group (isoflavone daily) or a placebo group, evaluating the effects of soy isoflavone administration for six months since the first treatment session. The authors observed reduced urinary incontinence, urgency, and better erectile function in the therapy group compared to the placebo at month three. The results in the sixth month were better, with less urinary leakage, fewer bowel changes as cramps, diarrhea, and less pain on defecation. Greater overall erection capacity was also observed, confirming the role in reducing discomfort, improving quality of life, and suggesting the prophylactic administration of soy isoflavone concomitant with radiotherapy in prostate cancer45.
In 2020, Crumbaker et al. evaluated the activity of idronoxil, a synthetic flavonoid derived from genistein with radiosensitizing properties, in association with a Lutetium-177 carrier molecule, a radiopharmaceutical, in 32 patients with metastatic castration-resistant prostate cancer. The flavonoid was administered rectally. The authors observed good tolerance to the drug combination, no serious adverse effects, high response rates to treatment, and improvement of pain in more than half of the patients with significant pain complaints at the beginning of the study50.
Zhao et al.47, in a phase I study, evaluated the administration of epigallocatechin gallate (EGCG), a polyphenol abundant in tea extracts, with antioxidant function, in 24 patients with stage III non-small cell lung carcinoma undergoing chemoradiotherapy with a diagnosis of dysphagia. Doses were administered immediately after identification of grade 2 dysphagia until two weeks after completion of radiotherapy. The authors observed a reduction of esophagitis and pain complaints in most of the patients47. The same group prospectively evaluated the administration of the same drug orally, in a phase II study, during radiotherapy and two weeks after completion, in 37 patients under the same conditions as in phase I and observed a significant reduction of pain and radiotherapy-induced acute esophagitis with p < 0.001. The solution was well tolerated by patients who reported only nausea related to the taste of the medication48.
Also, epigallocatechin gallate was evaluated in a randomized phase II study in 83 patients with non-small cell lung carcinoma, randomly assigned to three groups: prophylactic (EGCG at the start of radiotherapy), therapeutic, after the occurrence of esophagitis (EGCG after identification of grade 1 esophagitis up to two weeks after the end of radiotherapy) or conventional therapeutic (lidocaine, dexamethasone, and gentamicin after identification of grade 1 esophagitis up to two weeks after the end of radiotherapy). The degrees of esophagitis, pain and dysphagia were evaluated. The authors observed that treatment with EGCG three times daily showed better effects when compared to traditional treatment with lidocaine, dexamethasone, and gentamicin. In addition, prophylactic administration of the drug (at the beginning of radiotherapy) had a slight advantage over therapeutic administration in the treatment of radiotherapy-induced acute esophagitis44. Recently, in 2021, a study with a five-year survival analysis evaluated 38 patients with small-cell lung carcinoma and found that esophagitis and pain rates in patients receiving EGCG were lower than conventional treatment (p < 0.001), without reducing survival54.
Epigallocatechin gallate has also been the subject of studies on breast tumors. It was applied topically to 24 mastectomized patients after receiving adjuvant radiotherapy. The study noted that eight patients developed grade 2 dermatitis during or after radiotherapy, with regression to grade 1 after EGCG treatments. EGCG-associated acute erythema was observed in one patient. Reported symptoms (pain, burning, itching, and tenderness) decreased significantly (p < 0.05) two weeks after the end of radiotherapy41. A second, more robust study by the same team evaluated 49 patients with similar conditions and found that topical application of EGCG reduced pain in 85.7%, burning sensation in 89.8%, pruritus in 87.8%, pulling in 71.4%, and skin sensitivity in 79.6% of the patients51. A randomized, double-blind, clinical trial evaluated 165 breast cancer patients undergoing postoperative radiation therapy who received EGCG or placebo from the first day of radiation until two weeks after completion. The authors observed that the appearance of grade 2 or worse radiodermatitis was significantly lower in the EGCG group compared to placebo and that there was a significant reduction in symptom indices in the EGCG group, confirming its positive performance as a prophylactic agent43.
Recently, the effects of epigallocatechin gallate were evaluated in 20 head and neck cancer patients diagnosed with radiotherapy-induced mucositis. The compound was used in solution as a mouthwash three times daily. The authors evaluated mucosal toxicity, patient satisfaction, and mucositis-related pain on a weekly basis and found a significant reduction in mucositis-related pain (p < 0.05) and oral mucosal lesions, making it feasible to administer as a mouthwash40.
A randomized study with 40 patients with head and neck cancer was conducted to evaluate the potential of calendula extract to prevent and treat radiotherapy or chemoradiotherapy associated mucositis. The patients received Calendula officinalis, an extract rich in quercetin, every 12 hours by mouthwash. Statistical analysis was performed on 38 patients. The authors were able to conclude that Calendula extract was significantly effective in treating chemoradiation-induced mucositis compared to placebo from the second week onwards with good tolerance by the patients, however, it cannot completely prevent its occurrence49.
In 2020, Li et al.42 evaluated EGCG in esophageal cancer patients undergoing chemoradiotherapy or definitive radiotherapy with a diagnosis of radiation-induced esophagitis. The compound was administered orally, after diagnosis of esophagitis until two weeks after completion of radiation therapy. In addition, esophagitis-related dysphagia and pain were monitored weekly. The results showed a significant reduction in symptoms associated with radiotherapy-induced esophagitis, indicating EGCG as a protective agent in esophageal cancer.
In a randomized, double-blind phase II clinical trial, Yap et al.53 evaluated the effects of sinecatechins in 26 patients with vulvar intraepithelial neoplasia. Treatment was performed with topical application three times daily for 16 weeks, with follow-up for 52 weeks. In addition to the histological response, the authors also evaluated the degree of toxicity, quality of life, and pain score. As a result, sinecatechins showed better clinical response compared to placebo, with significant improvement in lesion resolution (p < 0.002). Furthermore, pain symptoms reduced after 16 weeks of treatment, compared to the beginning of the study, showing improvement in the quality of life of the patients.
The toxicity assessment was based on the CTCAE, a widely used global tool for grading adverse events81,82. Also, radiotherapy-induced cutaneous toxicity was analyzed using the RTOG criteria, a well-established and clinically recognized instrument83. The studies observed minimal to no adverse effects associated with the oral or topical administration of flavonoids, with reports limited to mild burning sensations and nausea, which were well tolerated throughout the treatment.
Pain assessment was performed using the Verbal Numeric Scale, a validated method selected by the studies investigated, that demonstrates high sensitivity and efficiency, applied to patients over 18 years of age and outside the context of sedation84. The complaint of pain was significantly lower in the groups treated with flavonoids during regular follow-up, highlighting the analgesic potential of the compounds used.
These measurement tools, globally recognized and widely utilized, are instrumental in guiding healthcare professionals in their clinical decision-making. Establishing a common communication approach is essential for determining the most beneficial interventions for the patient, particularly in managing pain, which is one of the primary complaints of cancer patients.
The assessment of the risk of bias is essential to determine the quality of the evidence and to demonstrate which aspects are methodologically weak based on analysis of the categories of the studies. The creation of a positive control group, with standard medication for pain control compared to the proposed treatment, would promote adherence and reduce the main bias identified.
The bias in the measurement of the outcome was identified in non-randomized studies. Therefore, the evaluation of the final results should be done by an individual who holds no relation with the study design or with its participants, providing safer blinding and reducing this type of bias. In this case, a double-blind design for randomized clinical trials would reduce the risk of bias, making the study more robust and validating the hypothesis suggested.
Thus, flavonoids seem to be promising molecules in pain management, as chemotherapy-associated neuropathy and tumor invasion-related pain. By reducing or eliminating the side effects of cancer therapies through the use of natural compounds, patient experience, quality of life, and treatment compliance are improved. However, more clinical trials and pre-clinical studies evaluating the potential of flavonoids in the pain associated with cancer itself, which is the most frequent, should be encouraged, with detailed pharmacological and other molecule analyses to expand their use and bring benefits to the patients.
CONCLUSION
Evidence of low risk of bias points to flavonoids as a good alternative in pain adjuvancy, especially in treatment-associated pain as radiodermatitis and mucositis in different topographies.
Flavonoids are naturally occurring compounds found in plants. In addition to their well-established anti-inflammatory potential, their application in the management of pain associated with oncological treatments has demonstrated mild adverse effects.
Numerous beneficial effects of flavonoid use in the context of oncology are being increasingly recognized and revisited. However, further research is required for thorough investigation of the analgesic potential of flavonoids, particularly in the context of cancer pain resulting from tumor invasion or metastasis. Moreover, it is necessary to expand the number of double-blind randomized controlled trials to minimize the risk of bias.
CONTRIBUTIONS
Maycon Giovani Santana and Matheus Cavalcante De Deus contributed substantially to the study design, Maycon Giovani Santana and Rachel Verdan Dib contributed to acquisition, analysis and interpretation of the data, Ana Lúcia Teodoro and Denise Gonçalves Priolli contributed to the wording and/or critical review. All the authors approved the final version for publication.
DECLARATION OF CONFLICT OF INTERESTS
There is no conflict of interests to declare.
FUNDING SOURCES
None.
REFERENCES
1. Livshits Z, Rao RB, Smith SW. An approach to chemotherapy-associated toxicity. Emerg Med Clin North Am. 2014;32(1):167-203.
2. Fallon MT. Neuropathic pain in cancer. Br J Anaesth. 2013;111(1):105-11.
3. Bennett M, Paice JA, Wallace M. Pain and opioids in cancer care: benefits, risks, and alternatives. Am Soc Clin Oncol Educ Book. 2017;37:705-13. doi: https://doi.org/10.1200/edbk_180469
4. Breivik H, Cherny N, Collett B, et al. Cancer-related pain: a pan-european survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20(8):1420-33.
5. Regan JM, Peng P. Neurophysiology of cancer pain. Cancer Control. 2000;7(2):111-9.
6. Simone CB, Vapiwala N, Hampshire MK,E et al. Cancer patient attitudes toward analgesic usage and pain intervention. Clin J Pain. 2012;28(2):157-62. doi: http://dx.doi.org/10.1097/ajp.0b013e318223be30
7. Deandrea S, Montanari M, Moja L, et al. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-91.
8. van Ryn M, Phelan SM, Arora NK, et al. Patient-reported quality of supportive care among patients with colorectal cancer in the veterans affairs health care system. J Clin Oncol. 2014;10;32(8):809-15. doi: https://doi.org/10.1200/jco.2013.49.4302
9. Neufeld NJ, Elnahal SM, Alvarez RH. Cancer pain: a review of epidemiology, clinical quality and value impact. Future Oncol. 2017;13(9):833-41.
10. Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. 2018;68(3):182-96. doi: https://doi.org/10.3322/caac.21453
11. Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34(27):3325-45. doi: https://doi.org/10.1200/jco.2016.68.5206
12. Correa D, Farney RJ, Chung F. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. 2015;120(6):1273-85 doi: https://doi.org/10.1213/ane.0000000000000672
13. Lee M, Silverman SM, Hansen H. A comprehensive review of opioid-induced hyperalgesia. Pain Physician [Internet]. 2011 [cited 2024 dec 20]:14(2):145-61. Available from: https://pubmed.ncbi.nlm.nih.gov/21412369/
14. Devulder J, Jacobs A, Richarz U. Impact of opioid rescue medication for breakthrough pain on the efficacy and tolerability of long-acting opioids in patients with chronic non-malignant pain. Br J of Anaesth. 2009;103(4):576-85. doi: https://www.doi.org/10.1093/bja/aep253
15. Matic I, Revandkar A, Chen J, et al. Identification of salvia haenkei as gerosuppressant agent by using an integrated senescence-screening assay. Aging. 2016;1;8(12):3223-40. doi: https://www.doi.org/10.18632/aging.101076
16. Argyropoulou A, Aligiannis N, Trougakos IP, et al. Natural compounds with anti-ageing activity. Nat Prod Rep. 2013;30(11):1412-37.
17. Dhiman A, Nanda A, Ahmad S. A quest for staunch effects of flavonoids: utopian protection against hepatic ailments. Arab J Chem. 2016;9(Sup2):S1813-23. doi: http://dx.doi.org/10.1016/j.arabjc.2012.05.001
18. Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513-23.
19. Orfali GC, Duarte AC, Bonadio V, et al. Review of anticancer mechanisms of isoquercitin. World J Clin Oncol. 2016;7(2):189-99.
20. Ren W, Qiao Z, Wang H, et al. Flavonoids: promising anticancer agents. ChemInform. 2003;34(38). doi: http://dx.doi.org/10.1002/chin.200338244
21. Li Y, Fang H, Xu W. Recent advance in the research of flavonoids as anticancer agents. Mini Rev Med Chem. 2007;7(7):663-78.
22. Begum AN, Terao J. Protective effect of quercetin against cigarette tar extract-induced impairment of erythrocyte deformability. J Nutr Biochem. 2002;13(5):265-72.
23. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13(10):572-84. doi: http://dx.doi.org/10.1016/s0955-2863(02)00208-5
24. Shimada Y, Dewa Y, Ichimura R, et al. Antioxidant enzymatically modified isoquercitrin suppresses the development of liver preneoplastic lesions in rats induced by beta-naphthoflavone. Toxicology. 2010;268(3):213-8.
25. Jung SH, Kim BJ, Lee EH, et al. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem Int. 2010;57(7):713-21.
26. Amado NG, Fonseca BF, Cerqueira DM, et al. Flavonoids: potential Wnt/beta-catenin signaling modulators in cancer. Life Sci. 2011;89(15-16):545-54.
27. Kim BH, Choi JS, Yi EH, et al. Relative antioxidant activities of quercetin and its structurally related substances and their effects on NF-κB/CRE/AP-1 signaling in murine macrophages. Mol Cells. 2013;35(5):410-20.
28. Bombardi Duarte AC, Santana MG, Camilo Orfali G, et al. Literature evidence and arrive assessment on neuroprotective effects of flavonols in neurodegenerative diseases’ Models. CNS Neurol Disord Drug Targets. 2018;17(1):34-42.
29. Santana MG, Orfali GC, Palma JKY, et al. Isoquercetina como novo alvo para inibição da angiogênese no câncer de cólon. J Coloproctology. 2019;39:214.
30. Makino T, Kanemaru M, Okuyama S, et al. Anti-allergic effects of enzymatically modified isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglucosyl rutin, and quercetin, when administered orally to mice. J Nat Med. 2013;67(4):881-6.
31. Rogerio AP, Kanashiro A, Fontanari C, et al. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007;56(10):402-8.
32. Araújo ME, Ye MF, Alberto TG, et al. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013;141(1):266-73. doi: https://doi.org/10.1016/j.foodchem.2013.02.127
33. Oliveira CTP, Colenci R, Pacheco CC, et al. Hydrolyzed rutin decreases worsening of anaplasia in glioblastoma relapse. CNS Neurol Disord Drug Targets. 2019;18(5)405-12. doi: https://doi.org/10.2174/1871527318666190314103104
34. Franco YEM, Lima CA, Rosa MN, et al. Investigation of U-251 cell death triggered by flavonoid luteolin: towards a better understanding on its anticancer property against glioblastomas. Nat Prod Res. 2021;35(22). doi: https://doi.org/10.1080/14786419.2020.1727470
35. Silva DC, Orfali GDC, Santana MG, et al. Antitumor effect of isoquercetin on tissue vasohibin expression and colon cancer vasculature. Oncotarget. 2022;13:307-18. doi: https://doi.org/10.18632/oncotarget.28181
36. University of York. Centre for Reviews and Dissemination. New York: University of York; 2019. PROSPERO - International prospective register of systematic reviews. 2023. [acesso 2024 set 24]. Disponível em: https://www.crd.york.ac.uk/PROSPERO/
37. Moher D. Preferred reporting items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Inter Med. 2009;151(4):264. doi: http://dx.doi.org/10.7326/0003-4819-151-4-200908180-00135
38. Higgins J, Welch V. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. [cited 2022 Dec 8]. Available from: www.handbook.cochrane.org
39. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: https://doi.org/10.1136/bmj.i4919
40. Zhu W, Mei H, Jia L, et al. Epigallocatechin-3-gallate mouthwash protects mucosa from radiation-induced mucositis in head and neck cancer patients: a prospective, non-randomised, phase 1 trial. Investigational new drugs. 2020;38(4):1129-36. doi: https://doi.org/10.1007/s10637-019-00871-8
41. Zhao H, Zhu W, Jia L, et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br J Radiol. 2016;89(1058):20150665. doi: https://doi.org/10.1259/bjr.20150665
42. Li X, Xing L, Zhang Y, et al. Phase II trial of epigallocatechin-3-gallate in acute radiation-induced esophagitis for esophagus cancer. J Med Food. 2020;23(1):43-9.
43. Zhao H, Zhu W, Zhao X, et al. Efficacy of epigallocatechin-3-gallate in preventing dermatitis in patients with breast cancer receiving postoperative radiotherapy: a double-blind, placebo-controlled, phase 2 randomized clinical trial. JAMA Dermatology. 2022;158(7):779.
44. Zhao H, Jia L, Chen G, et al. A prospective, three-arm, randomized trial of EGCG for preventing radiation-induced esophagitis in lung cancer patients receiving radiotherapy. Radiother Oncol. 2019;137:186-91.
45. Ahmad IU, Forman JD, Sarkar FH, et al. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutrition and cancer. 2010;62(7):996-1000. doi: https://doi.org/10.1080/01635581.2010.509839
46. Thomas R, Williams M, Cauchi M, et al. A double-blind, randomised trial of a polyphenolic-rich nail bed balm for chemotherapy-induced onycholysis: the UK polybalm study. Breast Cancer Res Treat. 2018;171(1):103-10. doi: https://doi.org/10.1007/s10549-018-4788-9
47. Zhao H, Zhu W, Xie P, et al. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother Oncol. 2014;110(1):132-6.
48. Zhao H, Xie P, Li X, et al. A prospective phase II trial of EGCG in treatment of acute radiation-induced esophagitis for stage III lung cancer. Radiother Oncol. 2015;114(3):351-6. doi: https://doi.org/10.1016/j.radonc.2015.02.014
49. Babaee N, Moslemi D, Khalilpour M, et al. Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: a randomized controlled clinical study. Daru. 2013;21(1):18. doi: https://doi.org/10.1186/2008-2231-21-18
50. Crumbaker M, Pathmanandavel S, Yam AO, et al. Phase I/II Trial of the combination of 177lutetium prostate specific membrane antigen 617 and idronoxil (NOX66) in men with end-stage metastatic castration-resistant prostate cancer (LuPIN). Eur Urol Oncol. 2021;4(6). doi: https://doi.org/10.1016/j.euo.2020.07.002
51. Zhu W, Jia L, Chen G, et al. Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy. Oncotarget. 2016;7(30):48607-13.
52. Kooshyar MM, Mozafari PM, Amirchaghmaghi M, et al. A randomized placebo- controlled double blind clinical trial of quercetin in the prevention and treatment of chemotherapy-induced oral mucositis. J Clin Diagn Res. 2017;11(3):ZC46-50.
53. Yap J, Slade D, Goddard H, et al. Sinecatechins ointment as a potential novel treatment for usual type vulval intraepithelial neoplasia: a single-centre double-blind randomised control study. BJOG. 2021;128(6):1047-55.
54. Zhu W, Zhao Y, Zhang S, et al. Evaluation of epigallocatechin-3-gallate as a radioprotective agent during radiotherapy of lung cancer patients: a 5-year survival analysis of a phase 2 study. Front Oncol. 2021;11:686950. doi: https://doi.org/10.3389/fonc.2021.686950
55. Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr Oncol. 2006;13(1):14-26.
56. Yao H, Xu W, Shi X, Zhang Z. Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29(1):1-31. doi: https://doi.org/10.1080/10590501.2011.551317
57. Swarm RA, Paice JA, Anghelescu DL, et al. Adult cancer pain, version 3. 2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(8):977-1007.
58. Lara-Solares A, Ahumada OM, Alá BP, et al. Latin-American guidelines for cancer pain management. Pain Manag. 2017;7(4). doi: https://doi.org/10.2217/pmt-2017-0006
59. Ventafridda V, Saita L, Ripamonti C, et al. WHO guidelines for the use of analgesics in cancer pain. Int J Tissue React. 1985;7(1):93-6.
60. Li Z, Zhang J, Ren X, et al. The mechanism of quercetin in regulating osteoclast activation and the PAR2/TRPV1 signaling pathway in the treatment of bone cancer pain. Int J Clin Exp Pathol. 2018;11(11):5149-56.
61. Britti D, Crupi R, Impellizzeri D, et al. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet Res. 2017;13(1):229.
62. Gao W, Zan Y, Wang ZJJ, et al. Quercetin ameliorates paclitaxel-induced neuropathic pain by stabilizing mast cells, and subsequently blocking PKCε-dependent activation of TRPV1. Acta Pharmacol Sin. 2016;37(9):1166-77.
63. Anjaneyulu M, Chopra K. Quercetin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J Exp Biol. 2004;42(8):766-9.
64. Çivi S, Emmez G, Dere ÜA, et al. Effects of quercetin on chronic constriction nerve injury in an experimental rat model. Acta Neurochir (Wien). 2016;158(5):959-65.
65. Spencer JPE. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010t;104(Suppl 3):S40-7.
66. Marder M, Paladini AC. GABA(A)-receptor ligands of flavonoid structure. Curr Top Med Chem. 2002;2(8):853-67.
67. Kanazawa LKS, Vecchia DD, Wendler EM, et al. Quercetin reduces manic-like behavior and brain oxidative stress induced by paradoxical sleep deprivation in mice. Free Radic Biol Med. 2016;99:79-86.
68. Li Q, Zhang X. Epigallocatechin-3-gallate attenuates bone cancer pain involving decreasing spinal Tumor Necrosis Factor-α expression in a mouse model. Int Immunopharmacol. 2015;29(2):818-23. doi: https://doi.org/10.1016/j.intimp.2015.08.037
69. Filho AW, Filho VC, Olinger L, et al. Quercetin: further investigation of its antinociceptive properties and mechanisms of action. Arch Pharm Res. 2008;31(6):713-21.
70. Hossain R, Al-Khafaji K, Khan RA, et al. Quercetin and/or ascorbic acid modulatory effect on phenobarbital-induced sleeping mice possibly through gaba and gaba receptor interaction pathway. Pharmaceuticals (Basel). 2021;14(8):721. doi: http://dx.doi.org/10.3390/ph14080721
71. Calixto-Campos C, Corrêa MP, Carvalho TT, et al. Quercetin reduces Ehrlich tumor-induced cancer pain in mice. Anal Cell Pathol (Amst). 2015;2015:285708. doi: https://doi.org/10.1155/2015/285708
72. Liu C, Liu DQ, Tian YK, et al. The emerging role of quercetin in the treatment of chronic pain. Current Neuropharmacology. 2022;20(12):2346-53.
73. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-63.
74. Semis HS, Kandemir FM, Kaynar O, et al. The protective effects of hesperidin against paclitaxel-induced peripheral neuropathy in rats. Life Sci. 2021;287:120104. doi: https://doi.org/10.1016/j.lfs.2021.120104
75. Gui Y, Zhang J, Chen L, et al. Icariin, a flavonoid with anti-cancer effects, alleviated paclitaxel-induced neuropathic pain in a SIRT1-dependent manner. Mol Pain. 2018;14:1744806918768970. doi: https://doi.org/10.1177/1744806918768970
76. Di Cesare Mannelli L, Zanardelli M, Failli P, et al. Oxaliplatin-induced neuropathy: oxidative stress as pathological mechanism. Protective effect of silibinin. J Pain. 2012;13(3):276-84. doi: https://doi.org/10.1016/j.jpain.2011.11.009
77. Azevedo MI, Pereira AF, Nogueira RB, et al. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol Pain. 2013;9:53. doi: https://doi.org/10.1186/1744-8069-9-53
78. Jiang W, Wang Y, Sun W, et al. Morin suppresses astrocyte activation and regulates cytokine release in bone cancer pain rat models. Phytother Res. 2017;31(9):1298-304. doi: https://doi.org/10.1002/ptr.5849
79. Wang A, Guo D, Cheng H, et al. Transcriptome sequencing explores the mechanism of baicalin on bone cancer pain. J Inflamm Res. 2021;14:5999-6010. doi: https://doi.org/10.2147/jir.s336028
80. Zhou YS, Cui Y, Zheng JX, et al. Luteolin relieves lung cancer-induced bone pain by inhibiting NLRP3 inflammasomes and glial activation in the spinal dorsal horn in mice. Phytomedicine. 2022;96:153910. doi: https://doi.org/10.1016/j.phymed.2021.153910
81. Minasian LM, O’Mara A, Mitchell SA. Clinician and patient reporting of symptomatic adverse events in cancer clinical trials: using CTCAE and PRO-CTCAE® to provide two distinct and complementary perspectives. Patient Relat Outcome Meas. 2022;13:249-58. doi: https://doi.org/10.2147/prom.s256567
82. Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the common terminology criteria for adverse events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas dermo-sifiliograficas [Internet]. 2021;112(1):90-2. doi: https://doi.org/10.1016/j.ad.2019.05.009
83. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341-6.
84. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798-804. doi: https://doi.org/10.1111/j.1365-2702.2005.01121.x
Recebido em 7/10/2024
Aprovado em 14/2/2025
Associate-editor: Fernando Lopes Tavares de Lima. Orcid iD: https://orcid.org/0000-0002-8618-7608
Scientific-editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777
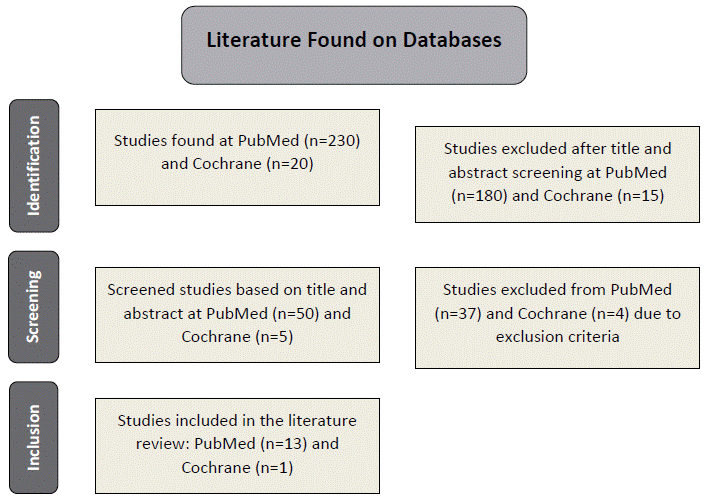
Figure 1. Flowchart of the selection of studies for the review based on the inclusion and exclusion criteria
Source: The authors, based on PRISMA37.
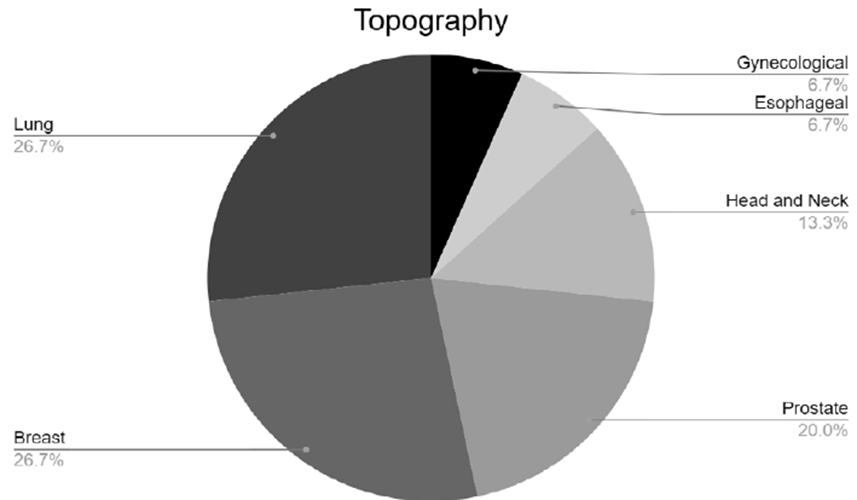
Figure 2. Percentage of cancer topographies analyzed by the studies included
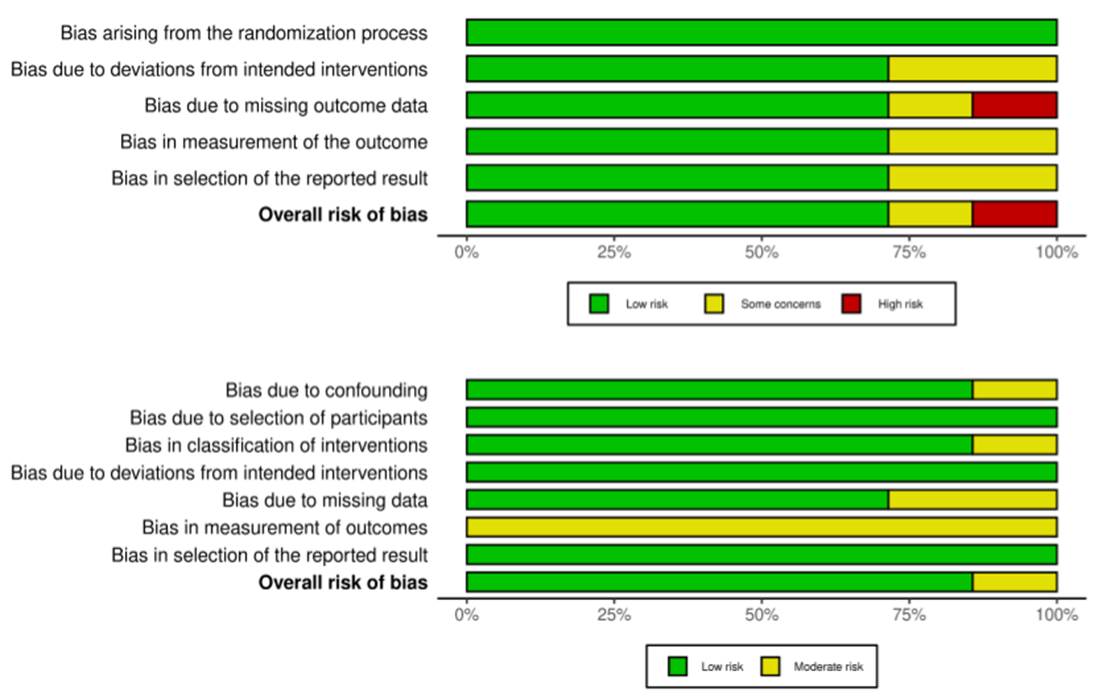
Figure 3. Risk of bias analysis performed by the Cochrane Collaboration Risk of Bias Tool (RoB 2.0 and ROBINS-I, respectively)
Table 1. Characteristics of the studies included, 2020-2024
|
Author |
Compound |
N* |
Site |
Design |
Conclusion |
|
|
Epigallocatechin-3-gallate mouthwash protects mucosa from radiation-induced mucositis in head and neck cancer patients: a prospective, non-randomised, phase 1 trial (2020) |
Zhu W, et al.40 |
Epigallocatechin |
20 |
Head and neck cancer |
Non-randomized |
EGCG administration reduces radiation-induced oral mucosal injury in patients |
|
Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy (2016) |
Zhao H, et al.41 |
Epigallocatechin |
24 |
Breast cancer |
Non-randomized |
Patient-reported symptom scores were significantly decreased in two weeks after the end of radiotherapy in pain, burning, itching and tenderness, p < 0.05 |
|
Phase II Trial of Epigallocatechin-3-Gallate in Acute Radiation-Induced Esophagitis for Esophagus Cancer (2020) |
Li X, et al.42 |
Epigallocatechin |
51 |
Esophageal cancer |
Non-randomized |
EGCG might be an acute radiation-induced esophagitis-reliever without compromising the efficacy of radiation therapy |
|
Efficacy of Epigallocatechin-3-Gallate in Preventing Dermatitis in Patients with Breast Cancer Receiving Postoperative Radiotherapy: A Double-Blind, Placebo-Controlled, Phase 2 Randomized Clinical Trial (2022) |
Zhao H, et al.43 |
Epigallocatechin |
180 |
Breast cancer |
Double-blind randomized |
Prophylactic use of EGCG solution significantly reduced the incidence and severity of radio-induced dermatitis in patients receiving adjuvant radiotherapy for breast cancer |
|
A prospective, three-arm, randomized trial of EGCG for preventing radiation-induced esophagitis in lung cancer patients receiving radiotherapy (2019) |
Zhao H, et al.44 |
Epigallocatechin |
83 |
Non-small-cell lung cancer |
Randomized controlled
|
The application of EGCG could effectively alleviate acute radiation esophagitis in advanced lung cancer without obvious side effects. Prophylactic application of EGCG had a slight advantage over therapeutic use in treatment of acute esophagitis |
|
Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer (2010) |
Ahmad IU, et al.45 |
Soy isoflavone |
27 |
Prostate cancer |
Double-blind randomized |
The results suggest that soy isoflavones taken in conjunction with radiation therapy could reduce the urinary, intestinal, and sexual adverse effects in patients with prostate cancer including less pain with bowel movements |
|
A double-blind, randomized trial of a polyphenolic-rich nail bed balm for chemotherapy-induced onycholysis: the UK polybalm study (2018) |
Thomas R, et al.46 |
Plant balm |
60 |
Breast and prostate cancer |
Double-blind, randomized |
The polyphenolic-rich essential oils reduced chemotherapy-related nail damage and improved nail-related quality of life, compared to a control |
|
A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer (2014) |
Zhao H, et al.47 |
Epigallocatechin |
24 |
Non-small-cell lung cancer |
Non-randomized |
The oral administration of EGCG is feasible, safe and effective, with dramatic regression of esophagitis to grade 0/1 and reduced pain score |
|
A prospective phase II trial of EGCG in treatment of acute radiation-induced esophagitis for stage III lung cancer (2015) |
Zhao H, et al.48 |
Epigallocatechin |
37 |
Lung cancer |
Non-randomized |
The oral administration of EGCG is an effective and safe method to deal with acute radiation-induced esophagitis |
|
Antioxidant capacity of calendula officinalis flowers extract and prevention of radiation induced oropharyngeal mucositis in patients with head and neck cancers: a randomized controlled clinical study (2013) |
Babaee N, et al.49 |
Calendula extract (quercetin) |
40 |
Head and neck cancer |
Double-blind randomized |
Calendula extract was effective in decreasing the intensity of radiotherapy-induced mucositis, and the antioxidant capacity of quercetin may be partially responsible for the effect |
|
Phase I/II Trial of the Combination of 177Lutetium Prostate specific Membrane Antigen 617 and Idronoxil (NOX66) in Men with End-stage Metastatic Castration-resistant Prostate Cancer (LuPIN) (2021) |
Crumbaker M, et al.50 |
Idronoxil (genistein) |
32 |
Prostate cancer |
Non-randomized |
Idronoxil with LuPSMA-617 is a safe and feasible therapeutic strategy, with pain improvement in men treated with third-line therapy for prostate cancer
|
|
Epigallocatechin-3-gallate ameliorates radiation-induced acute skin damage in breast cancer patients undergoing adjuvant radiotherapy (2016) |
Zhu W, et al.51 |
Epigallocatechin |
49 |
Breast cancer |
Non-randomized |
EGCG reduced the pain in 85.7% of patients, burning-feeling in 89.8%, itching in 87.8%, pulling in 71.4%, and tenderness in 79.6% |
|
A Randomized Placebo- Controlled Double Blind Clinical Trial of Quercetin in the Prevention and Treatment of Chemotherapy-Induced Oral Mucositis (2017) |
Kooshyar MM, et al.52 |
Quercetin |
23 |
Hematologic cancer |
Double-blind, randomized |
The incidence of mucositis was lower in the quercetin group. Mucositis was more severe in the intervention group, which may be due to lower oral health status. |
|
Sinecatechins ointment as a potential novel treatment for usual type vulval intraepithelial neoplasia: a single-centre double-blind randomised control study (2020) |
Yap J, et al.53 |
Sinecatechins |
26 |
Vulvar intraepithelial neoplasia |
Double-blind randomized |
The application of sinecatechins is safe and shows promise in inducing clinical resolution of vulval intraepithelial neoplasia lesions and improving symptoms such as pain |
|
Evaluation of Epigallocatechin-3-Gallate as a Radioprotective Agent During Radiotherapy of Lung Cancer Patients: A 5-Year Survival Analysis of a Phase 2 Study (2021) |
Zhu W, et al.54 |
Epigallocatechin |
38 |
Small cell lung cancer |
Randomized controlled |
ECG can relieve some esophagitis-related scores, such as pain, in SCLC patients exposed to ionizing radiation without reducing survival |
(*) Sample.
![]()
Este é um artigo publicado em acesso aberto (Open Access) sob a licença Creative Commons Attribution, que permite uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o trabalho original seja corretamente citado.