ORIGINAL ARTICLE
Delays in Cervical Cancer Treatment Initiation and Associated Factors in
a Hospital-Based Cohort in the Brazilian Western Amazon
Atrasos no Início do Tratamento do
Câncer Cervical e Fatores Associados em uma Coorte Hospitalar da Amazônia Ocidental
Brasileira
Retrasos en el Inicio del Tratamiento
del Cáncer Cervical y Factores Asociados en una Cohorte Hospitalaria de la Amazonía
Occidental Brasileña
https://doi.org/10.32635/2176-9745.RBC.2025v71n4.5228
Liz Rodrigues de
Souza1; Maria Fernanda de Sousa Oliveira Borges2; Ilce
Ferreira da Silva3
1,2Universidade
Federal do Acre (UFAC), Programa de Pós-Graduação em Saúde Coletiva. Rio Branco
(AC), Brasil. E-mails: lizrodriguessouza@gmail.com;
maria.borges@ufac.br. Orcid iD: https://orcid.org/0009-0009-0539-0558;
Orcid iD: https://orcid.org/0000-0002-5536-6507
3UFAC.
Rio Branco (AC), Brasil. Fundação Oswaldo Cruz (Fiocruz), Escola Nacional de
Saúde Pública Sérgio Arouca (Ensp). Rio de Janeiro (RJ), Brasil. E-mail:
ilce23@hotmail.com. Orcid iD: https://orcid.org/0000-0002-7134-3030
Corresponding
author: Ilce Ferreira da Silva. Ensp/Fiocruz. Rua Leopoldo Bulhões, 1480 – 8º
andar, sala 821 – Manguinhos. Rio de Janeiro (RJ), Brasil. CEP 21041-210. E-mail: ilce23@hotmail.com
ABSTRACT
Introduction: Early diagnosis followed
by timely treatment of cervical cancer are essential for better prognosis and
survival. Objective: To estimate the prevalence of delay in
cervical cancer treatment initiation and associated factors in women diagnosed
and treated in Rio Branco-Acre between 2012 and 2017. Method:
Cross-sectional study of a hospital cohort involved women with cervical cancer
treated between 2012 and 2017 in Rio Branco, Acre. The time in days from
diagnosis to the first treatment was categorized according to the Brazilian
law. Student’s t and Kruskal-Wallis tests analyzed continuous variables, while
categorical variables were evaluated using X2-Pearson and Fisher tests, with a
5% significance level. Crude and adjusted prevalence ratios (OR) were estimated
by Poisson regression with robust variance, with 95% CI. Results:
The median time between diagnosis and first treatment was 39 days, ranging from
81 days for surgical treatment to 29 days for isolated radiotherapy. The
prevalence of delay in treatment initiation was 34%, and the factors associated
with the delay included being aged >40 years, waiting >30 days for specialist
consultation, and first surgical treatment. The radiotherapy with/without
brachytherapy treatment protocol and stages IIIB-IVA were inversely associated
with delay. Conclusion: Delay in cervical cancer treatment
initiation in Acre was shorter than in other regions of the country. Age >40
years, waiting >30 days for a specialist consultation were positively
associated with delay, while advanced stages were inversely associated.
Key words: Uterine Cervical
Neoplasms; Women's Health; Time-to-Treatment; Treatment Delay/statistics &
numerical data.
RESUMO
Introdução: O diagnóstico precoce seguido de tratamento em tempo
oportuno do câncer cervical são fundamentais para melhor prognóstico e
sobrevida. Objetivo: Estimar a prevalência do atraso no início do
tratamento do câncer cervical e os fatores associados em mulheres
diagnosticadas e tratadas em Rio Branco-Acre entre 2012 e 2017. Método:
Estudo transversal da coorte hospitalar de mulheres com câncer cervical
tratadas entre 2012 e 2017 em Rio Branco, Acre. O tempo entre o diagnóstico e o
primeiro tratamento foi categorizado conforme a legislação brasileira.
Variáveis contínuas foram analisadas pelos testes t de Student e Kruskal-Wallis,
enquanto variáveis categóricas foram avaliadas por X2-Pearson e Fisher, com
significância de 5%. As razões de prevalência (PR) brutas e ajustadas foram
calculadas por regressão de Poisson com variância robusta, com intervalo de
confiança de 95%. Resultados: O tempo mediano entre o diagnóstico
e o primeiro tratamento foi de 39 dias, variando de 81 dias para tratamento
cirúrgico a 29 dias para radioterapia isolada. A prevalência de atraso no
início do tratamento foi de 34%. Fatores associados ao atraso incluíram idade
acima de 40 anos e tempo >30 dias para consulta com especialista e primeiro
tratamento cirúrgico. O protocolo de tratamento radioterapia com e sem
braquiterapia e estadiamentos IIIB-IVA foram inversamente relacionados ao
atraso. Conclusão: O atraso no início do tratamento do câncer
cervical no Acre foi menor do que em outras localidades do país. Idade >40
anos e esperar >30 dias por consulta com especialista foram fatores
positivamente associados ao atraso, enquanto estadiamentos avançados foram
inversamente associados.
Palavras-chave: Neoplasias do Colo do Útero; Saúde
da Mulher; Tempo para o Tratamento; Atraso no tratamento/estatística &
dados numéricos.
RESUMEN
Introducción: El diagnóstico temprano
seguido de un tratamiento oportuno del cáncer cervical son fundamentales para
un mejor pronóstico y supervivencia. Objetivo: Estimar la
prevalencia del retraso en el inicio del tratamiento del cáncer cervical y los
factores asociados en mujeres diagnosticadas y tratadas en Río Branco, Acre,
entre 2012 y 2017. Método: Estudio transversal de la cohorte
hospitalaria de mujeres con cáncer cervical tratadas en Río Branco, Acre, entre
2012 y 2017. Se categorizó el tiempo entre el diagnóstico y el primer
tratamiento según la legislación brasileña. Las variables continuas fueron
analizadas con pruebas t de Student y Kruskal-Wallis, y las categóricas con
X2-Pearson y Fisher. Se calcularon razones de prevalencia (RP) brutas y
ajustadas mediante regresión de Poisson con varianza robusta, con intervalos de
confianza del 95%. Resultados: La mediana del tiempo mediano
entre el diagnóstico y el primer tratamiento fue de 39 días, con variaciones
entre 81 días para cirugía y 29 días para radioterapia aislada. La prevalencia
del retraso fue del 34%. Factores asociados al retraso incluyeron edad >40 años, espera >30 días para consulta con
especialista y tratamiento quirúrgico. La radioterapia con/sin braquiterapia y
los estadios IIIB-IVA estuvieron inversamente relacionados con el retraso. Conclusión:
El retraso en el inicio del tratamiento del cáncer cervical en Acre fue menor
que en otras regiones del país. Edad >40 años, esperar >30 días para consulta con especialista y
cirugía como primer tratamiento estuvieron asociados positivamente con el retraso,
mientras que los estadios avanzados estuvieron inversamente asociados.
Palabras clave: Neoplasias del Cuello Uterino; Tiempo
de Tratamiento; Salud de la Mujer; Retraso en el Tratamiento/estadística &
datos numéricos.
INTRODUCTION
Cervical cancer is
considered a serious public health problem, accounting for approximately
660,000 new cases and 348,000 deaths in women worldwide in 20221. In
Brazil, incidence rates vary by region, the highest incidence and mortality
rates were registered in the North and Northeast regions2,3.
According to the Population-Based Cancer Registry (PBCR), incidence rates
varied from 7.43 in Porto Alegre-RS (2013 to 2017) to 10.95 in Curitiba-PR
(2014 to 2018) in the South region, while in the North region, the incidence varied
from 28.98 in Amapá-AP (2016) to 38.25 in Manaus-AM (2012 to 2016)4.
In 2020, cervical cancer was the most common cancer among women in the same
region, accounting for 21% of all primary neoplasms4. In the state of Acre (Western Brazilian Amazon), the incidence of this
neoplasm, the second most common, was 41.3/100,000 women between 2007 and 20095.
Cervical cancer mortality
can be influenced by sociodemographic, clinical-epidemiological factors, tumor
characteristics (staging at diagnosis, histological type, tumor grade, among
others), and treatment type6-8, besides structural and
organizational aspects of access to diagnosis and waiting time between
diagnosis and treatment initiation8,9. Conservative surgical
procedures are preferred for young patients in stages IA2 and IB1 to preserve
ovaries and hormonal reproductive functions7,10,11 and adjuvant treatments
vary based on risk, medical history, and cancer size10-12. Advanced stages need more aggressive treatment.
The literature divides
delays in treatment initiation into ‘healthcare-related delays’ and
‘patient-related delays’13-15. The first would be
associated with difficulties or lack of access to health services, poor quality
of services provided, long waiting periods for treatment initiation and long
waiting lists, and the second, with low education, lack of resources for
transportation and other sociodemographic factors, poor knowledge about the
disease, seeking a second or third opinion, comorbidities, previous
experiences, and family history13,16.
Although some studies have
not identified a significant association between delay in treatment initiation
of cervical cancer and lower survival rates17-19, evidence indicates that
a period over 60 days between the diagnosis and the treatment initiation could
lead to a worse prognosis compared to women who were treated within 60 days
after diagnosis20-22. Furthermore, the lack of
association may be related to non-standardized waiting time among the studies13,23, the variation staging at diagnosis, small sample size, and
different study bases (hospital vs. population)11.
While international studies
contribute to the understanding of care intervals – the time between diagnosis
and the first specialist consultation or treatment initiation – they often do
not provide a standardized definition of what a “delay” is in oncological care.
Although they offer valuable references for understanding time management and
structuring care pathways, their findings are not directly comparable13,14,18,24.
In contrast, Brazil has a
legally established benchmark for oncological care: Law number 12,732/2012 ensures
free cancer treatment through the National Health System (SUS) that should
begin within 60 days of diagnosis25. This legal clarity not
only provides a normative standard for evaluating access to care but also
enables the development of studies that consistently define what constitutes a treatment
delay.
However, recent studies
suggest that the interval between diagnosis and treatment initiation still
varies according to the patient’s residence and type of tumor, often exceeding
the mandated deadline21,25-28.
Among these studies, the prevalence of delays varied from 65.1% in Bahia to
92.8% in Rio de Janeiro28,29. This variation may be
due to differences in study bases (hospital vs. population), which may
compromise the results due to sample size and representativeness30. In addition, oncology service organization varies among states and
regions, affecting the staging at diagnosis and the prevalence of delays21,29,31.
Moreover, a
population-based study revealed that over 70% of women diagnosed with cervical
cancer in the North region initiated treatment more than 60 days after
diagnosis. This high percentage remained stable until 2018 and only declined to
60% in 202032. These findings reinforce the
disparities in access to cancer care across regions and demonstrate the need
for localized investigations to better understand structural and systemic
barriers in different parts of the country.
Only one of these studies
assessed the factors associated with the delay in cervical cancer treatment
initiation28, and none of them was conducted in
the Western Brazilian Amazon. Therefore, this study aims to estimate the
prevalence of the delay in cervical cancer treatment initiation and associated
factors in women diagnosed and treated in Rio Branco-Acre, Western Brazilian
Amazon, between 2012 and 2017.
METHOD
This is a cross-sectional
study based on a cohort of women with cervical cancer treated from 2012 to 2017
in all hospital units authorized to provide oncological care in the city of Rio
Branco, Acre.
Women diagnosed with
cervical cancer treated between January 1, 2012, and December 31, 2017 at the
High Complexity Oncology Unit (Unacon) – “Hospital de Câncer de Acre” –
and at “Hospital Santa Juliana” were eligible for this study. Unacon is
the SUS reference state unit for oncological care in Acre and the only health
facility providing radiotherapy treatment in the state. “Hospital Santa
Juliana” is the reference private health network for gynecological
surgeries.
Women eligible for the
study were those diagnosed with primary cervical cancer according to the
International Statistical Classification of Diseases and Related Health
Problems 10th revision (ICD 10)33, codes C53, C53.0, C53.1,
C53.8, and C53.9. Eligible women were identified from Unacon’s Cancer Hospital
Registry databases and by evaluating the medical records of each woman who
underwent gynecological surgery at “Hospital Santa Juliana”. The
diagnosis was confirmed by their histopathological results.
Exclusion criteria were
women whose treatment protocol was defined and implemented outside Rio Branco,
who presented tumors other than epithelial, those who had neoplasms in a second
primary site, pregnant women who waited until the end of pregnancy to start
treatment, and those at stage IVB because the treatment was palliative and not
curative. Thus, among the 480 women identified according to ICD C53 in the
hospitals' databases, 54 (11.25%) were excluded. Of the 426 patients included
in the study, there were 23 losses (5.3%). Thus, the study population consisted
of 403 women with cervical cancer (Figure 1).
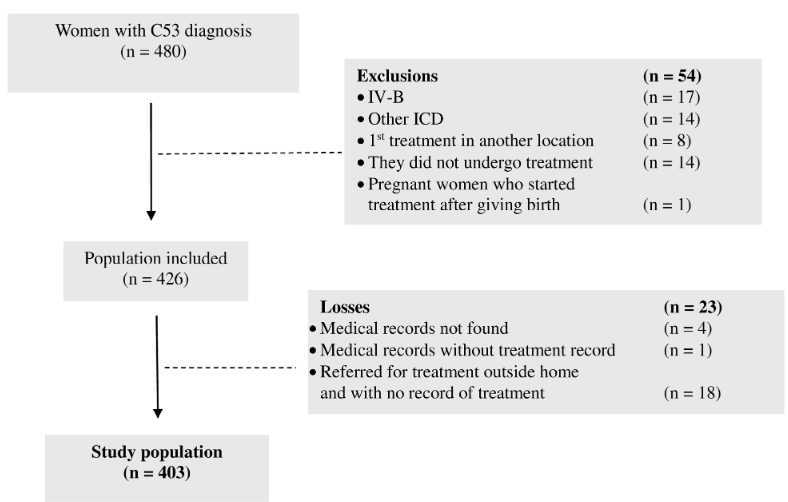
Figure 1. Eligibility
flowchart of the study population in Rio Branco, Acre (2012 to 2017)
Data were extracted from
physical medical records by a trained nurse, with a standardized form developed
for this purpose, with information covering socioeconomic and demographic data,
clinical history, family history of cancer, tumor characteristics, treatment
performed, diagnostic data, start and end data for each treatment implemented.
Treatment and staging were
found in patient records, with the stage following the recommendations of the
International Federation of Gynecology and Obstetrics (FIGO)10. Radiotherapy and
chemotherapy procedures were performed at Unacon, based on the treatment plan
established during the initial consultation with a specialist. In case of a
defective radiation device, these patients were referred through the
Out-of-Home Treatment (OHT) program for treatment at another institution, and
returned to Unacon to continue subsequent treatments or post-treatment
follow-up.
Since Unacon has no
surgical center, patients in the early stage of diagnosis were referred for
surgery outside of its facilities. The reference surgical center for
oncological surgeries is SUS’ Hospital de Clínicas do Acre (also known
as FUNDHACRE – Fundação Hospital Estadual do Acre) of which Unacon is
part of the hospital complex. For surgeries performed at “Hospital Santa
Juliana”, of the 448 women who underwent hysterectomy procedures between
2012 and 2017, only three were diagnosed with cervical cancer, and all three were
being followed-up at Unacon.
The dependent variable was
defined as the waiting time (in days) between the date of diagnosis and the
date of the first treatment, analyzed as a continuous variable and then
categorized into < 60 days and > 60 days, according to Law 12,732/201225.
A time-related variable
was derived from three specific dates collected from medical records: the
diagnosis date, defined as the date of histopathological confirmation; the
specialist consultation date is the first appointment with an oncology
specialist; and the treatment initiation date is the date of the first
therapeutic procedure (surgery, radiotherapy, or chemoradiotherapy).
Based on these dates, two
time intervals were calculated: the first (T1) refers to the number of days
between diagnosis and the first specialist consultation, categorized as <30
days and ≥30 days; the second (T2) corresponds to the interval between
diagnosis and treatment initiation, categorized as <60 days, 60–90 days, and
>90 days, and analyzed both as a continuous and a categorical variable.
The independent variables
included sociodemographic and economic characteristics (age at admission,
residence, skin color, education level, and marital status), lifestyle habits
(smoking and alcohol use), family history of cancer, clinical history/tumor
characteristics (comorbidities, histological type, FIGO (International
Federation of Gynecology and Obstetrics) stage10),
treatment performed (surgery only; surgery + radiation; radiation only;
chemoradiotherapy) and OHT, which refers to situations where women were
referred to other SUS network units to carry out part of the treatment due to
defects and maintenance in the radiation device.
Descriptive analyses were
performed using frequency distributions of variables according to the type of
treatment received. Percentage differences were assessed using Pearson's
Chi-square test (x2) and Fisher's tests, considering a significance level of
5%. Waiting times (between diagnosis and first specialist consultation and
diagnosis and treatment initiation) were analyzed both as continuous and
categorical variables, assigning them the times of <30, 31-60, >60
and <60, 61-90, >90 days. The distribution of time intervals
according to the first treatment and stage of diagnosis were analyzed using the
Kruskal-Wallis and Mann-Whitney tests, both with a significance level of 5%.
The prevalence of delay in treatment initiation was categorized for each
variable according to the stage at diagnosis. Crude prevalence ratios (PR) were
then estimated for all stages and stratified by stage, with 95% confidence
intervals, using Poisson regression with robust variance34,35. Multiple Poisson regression models were performed to estimate
adjusted prevalence ratios for delay in treatment initiation according to the
independent variables and their respective 95%CI using the Wald test.
Biological relevance and statistical significance in the crude analyses were
used as inclusion criteria in the final model. The final model underwent
residual analysis and assessment of model fit.
All analyses were
performed using the SPSS36 statistical package (version 20.0, SPSS,
Chicago, IL).
The Ethics Committee of ”Universidade
Federal do Acre” approved the study, report number 6,409,404 on October 6th,
2023, in compliance with Directive 466/2012 of the National Health Council,
CAAE (submission for ethical review) 74178923.1.0000.501037.
RESULTS
Of the 403 women included,
72% were aged 40 years or older and 54.3% lived outside Rio Branco. The
majority self-identified as Brown (80.9%), had completed elementary school
(40.5%), were married (47.8%) and had a history of smoking (62.5%). 94.3% were
diagnosed with squamous cell carcinoma and 78.4% with late stage (IIB or
higher) according to histological and staging characteristics. Of the women
included in the study, 46.7% underwent some part of the radiation treatment
outside their home through referral by “Hospital de Câncer do Acre” (Unacon)
to another public institution due to problems with the radiation equipment. The
first specialist consultation within a period of less than or equal to 30 days
from the date of diagnosis was observed in 69.7% of the women. In relation to
patients who underwent exclusive surgery, those who were treated with
chemoradiation were at more advanced stage of the disease (96.3%) (Table 1).
Table 1. Distribution of
characteristics of a cohort of women diagnosed with cervical cancer and treated
in Rio Branco, Acre (2012 to 2017)
|
Treatment Protocol
|
|
Characteristics
|
Total*
|
Exclusive surgery
|
Surgery + Radiation**
|
Exclusive radiation***
|
Chemoradiation
|
X² Test
|
|
|
N (%)
|
N (%)
|
N (%)
|
N (%)
|
N (%)
|
p-value
|
|
|
403 (100)
|
48 (100)
|
29 (100)
|
139 (100)
|
187 (100)
|
|
|
Age at
admission
|
|
0.037a
|
|
<40
|
113 (28.0)
|
14 (29.2)
|
11 (37.9)
|
27 (19.4)
|
61 (32.6)
|
|
|
>40
|
290 (72.0)
|
34 (70.8)
|
18 (62.1)
|
112 (80.6)
|
126 (67.4)
|
|
|
Residence
|
|
0.384a
|
|
Rio Branco
|
184 (45.7)
|
26 (54.2)
|
16 (55.2)
|
60 (43.2)
|
82 (43.9)
|
|
|
Others
|
219 (54.3)
|
22 (45.8)
|
13 (44.8)
|
79 (56.8)
|
105 (56.1)
|
|
|
Skin color
|
|
0.804b
|
|
Brown
|
263 (80.9)
|
36 (83.7)
|
19 (86.4)
|
81 (78.6)
|
127 (80.9)
|
|
|
Others
|
62 (19.1)
|
7 (16.3)
|
3 (13.6)
|
22 (21.4)
|
30 (19.1)
|
|
|
Education
|
|
0.000a
|
|
> High school
|
74 (23.8)
|
14 (37.8)
|
10 (50.0)
|
12 (11.2)
|
38 (25.9)
|
|
|
Elementary school
|
126 (40.5)
|
13 (35.1)
|
6 (30.0)
|
41 (38.3)
|
66 (44.9)
|
|
|
Illiterate
|
111 (35.7)
|
10 (27.0)
|
4 (20.0)
|
54 (50.5)
|
43 (29.3)
|
|
|
Marital
status
|
|
0.002b
|
|
Married/stable
union
|
183 (47.8)
|
29 (60.4)
|
16 (59.3)
|
46 (35.7)
|
92 (51.4)
|
|
|
Widow
|
60 (15.7)
|
5 (10.4)
|
3 (11.1)
|
35 (27.1)
|
17 (9.5)
|
|
|
Divorced/separated
|
28 (7.3)
|
1 (2.1)
|
1 (3.7)
|
11 (8.5)
|
15 (8.4)
|
|
|
Single
|
112 (29.2)
|
13 (27.1)
|
7 (25.9)
|
37 (28.7)
|
55 (30.7)
|
|
|
Comorbidities
|
|
0.000a
|
|
No
|
209 (59.0)
|
19 (55.9)
|
19 (79.2)
|
48 (39.3)
|
123 (70.7)
|
|
|
Yes
|
145 (41.0)
|
15 (44.1)
|
5 (20.8)
|
74 (60.7)
|
51 (29.3)
|
|
|
Smoking
history
|
|
0.020a
|
|
No
|
132 (37.5)
|
12 (37.5)
|
12 (48.0)
|
33 (26.8)
|
75 (43.6)
|
|
|
Yes
|
220 (62.5)
|
20 (62.5)
|
13 (52.0)
|
90 (73.2)
|
97 (56.4)
|
|
|
Alcohol
history
|
|
|
|
|
|
0.101a
|
|
No
|
182 (53.4)
|
16 (51.6)
|
13 (52.0)
|
72 (62.6)
|
81 (47.6)
|
|
|
Yes
|
159 (46.6)
|
15 (48.4)
|
12 (48.0)
|
43 (37.4)
|
89 (52.4)
|
|
|
Histological type
|
|
0.140b
|
|
Squamous cell
carcinoma
|
380 (94.3)
|
44 (91.7)
|
25 (86.2)
|
131 (94.2)
|
180 (96.3)
|
|
|
Adenocarcinoma
|
23 (5.7)
|
4 (8.3)
|
4 (13.8)
|
8 (5.8)
|
7 (3.7)
|
|
|
Stage
|
|
0.000b
|
|
IA1 – IIA2
|
87 (21.6)
|
48 (100)
|
13 (44.8)
|
19 (13.7)
|
7 (3.7)
|
|
|
IIB – IIIA
|
75 (18.6)
|
0 (0.0)
|
4 (13.8)
|
25 (18.0)
|
46 (24.6)
|
|
|
IIIB - IVA
|
241 (59.8)
|
0 (0.0)
|
12 (41.4)
|
95 (68.3)
|
134 (71.7)
|
|
|
OHT
|
|
|
|
|
|
0.000a
|
|
No
|
215 (53.3)
|
48 (100)
|
23 (79.3)
|
75 (54.0)
|
69 (36.9)
|
|
|
Yes
|
188 (46.7)
|
0 (0.0)
|
6 (20.7)
|
64 (46.0)
|
118 (63.1)
|
|
|
Time to first
specialist consultation
|
|
0.403a
|
|
< 30 days
|
281 (69.7)
|
31 (64.6)
|
17 (58.6)
|
101 (72.7)
|
132 (70.6)
|
|
|
> 30 days
|
122 (30.3)
|
17 (35.4)
|
12 (41.4)
|
38 (27.3)
|
55 (29.4)
|
|
*Totals may
vary due to missing values; **Radiation with or without chemotherapy;
***Radiation with or without brachytherapy; aChi-square test; bFisher's
exact test.
The median time between
diagnosis and the first specialist consultation was 18 days, 23 days for
surgery (alone or combined with radiation) and 17 days for radiotherapy and
chemoradiation. The stages at diagnosis varied between 22 days for IA1 and IIA2
and 17 days for IIIB and IVA while the majority of the women (66%) started
treatment within the 60-day range. A median of 39 days was identified, with 81
days of time interval for surgical treatment, 29 days for treatment with
radiation alone and 36 days for treatment with chemoradiation. According to the
stages, it is possible to identify a median of 84 days for initial stages (IA1
to IIA2), and 39 and 28 days for more advanced stages (IIB-IIIA and IIIB-IVA,
respectively) (Table 2).
The prevalence of delay in
treatment initiation was less than 50% for all variables evaluated, considering
all stages, except for the variables time between diagnosis and first
consultation with a specialist, in which the prevalence was 65.6% for times
greater than 30 days, first surgical treatment, in which the prevalence was
58.4% and first radiation treatment, where the delay was greater in 57.7% of
women who did not undergo radiotherapy treatment at first (Table 3).
Overall, age > 40 years
(PR= 1.83; 95%CI: 1.25-2.69), smoking history (PR= 1.42; 95% CI: 1.02-1.98),
alcohol use history (PR= 1.51; 95% CI: 1.12-2.03), time to first consultation
>30 days (PR= 3.23; 95% CI: 2.48-4.21) and first surgical treatment (PR=
2.07; 95% CI: 1.60-2.67) were positively associated with delayed treatment
initiation, while first radiotherapy treatment (PR= 0.49; 95% CI: 0.37-0.63)
was negatively associated with delayed treatment initiation. Women at IA1 to
IIA2 who underwent their first surgical treatment were positively associated
with delays (PR= 1.87; 95% CI: 1.12-3.12), while the same group of women who
underwent their first radiotherapy treatment were negatively associated with
delay (PR= 0.53; 95% CI: 0.32-0.88). At more advanced stages (IIIB to IVA),
women aged >40 years (PR= 2.61; 95%CI: 1.19-5.74), with a history of alcohol
use (PR= 1.78; 95%CI: 1.34-2.80) and waiting time between diagnosis and first
consultation >30 days (PR= 6.07; 95%CI: 3.78-9.75) were positively
associated with delays in treatment initiation (Table 3). It should be noted
that the stratification by stage in Table 3, although important for
understanding specific patterns of delay, resulted in reduced sample sizes
within each stratum, which may have compromised the precision of the estimates,
as indicated by the wider 95% confidence intervals.
Table 2. Distribution of the time
interval between women diagnosed with cervical cancer and treated in Rio
Branco, Acre, according to the first treatment, stage at diagnosis and part of
the treatment through OHT (2012 to 2017)
|
|
|
Treatment protocol
|
Stage at diagnosis
|
OHT
|
|
|
Estimate
|
Total*
|
Surgery**
|
Exclusive Radiotherapy***
|
Chemoradiation
|
X² Test
|
IA1-IIA2
|
IIB-IIIA
|
IIIB-IVA
|
X² Test
|
No
|
Yes
|
X² Test ²
|
|
|
N (%)
|
N (%)
|
N (%)
|
N (%)
|
p-value
|
N (%)
|
N (%)
|
N (%)
|
p-value
|
N (%)
|
N (%)
|
p-value
|
|
|
Total
|
403 (100)
|
77 (100)
|
139 (100)
|
187 (100)
|
|
87 (100)
|
75 (100)
|
241 (100)
|
|
215 (100)
|
188 (100)
|
|
|
|
Time between diagnosis and first specialist assessment
|
|
Mean (SD)
|
33.3 (52.5)
|
47.1 (7.4)
|
33.0 (5.0)
|
27.9 (2.8)
|
0.050 b
|
43.9 (6.7)
|
30.2 (6.2)
|
30.5 (3.0)
|
0.056 b
|
38.5 (65.1)
|
27.4 (31.7)
|
0.957c
|
|
|
Median
|
18
|
23
|
17
|
17
|
|
22
|
19
|
17
|
|
17
|
18
|
|
|
|
Min - Max
|
0 - 456
|
2 - 322
|
0 - 456
|
0 - 403
|
|
0 - 322
|
0 - 456
|
0 - 403
|
|
0 -456
|
0 – 280
|
|
|
|
< 30
|
281 (69.7)
|
48 (62.3)
|
101 (72.7)
|
132 (70.6)
|
0.201 a
|
54 (62.1)
|
54 (72.0)
|
173 (71.8)
|
0.221 a
|
146 (67.9)
|
135 (71.8)
|
0.142a
|
|
|
31-60
|
67 (16.6)
|
12 (15.6)
|
22 (15.8)
|
33 (17.6)
|
|
17 (19.5)
|
15 (20.0)
|
35 (14.5)
|
|
33 (15.3)
|
34 (18.1)
|
|
|
|
> 60
|
55 (13.6)
|
17 (22.1)
|
16 (11.5)
|
22 (11.8)
|
|
16 (18.4)
|
6 (8.0)
|
33 (13.7)
|
|
36 (16.7)
|
19 (10.1)
|
|
|
|
Time between diagnosis and first treatment initiation
|
|
Mean (SD)
|
63.6 (87.6)
|
112.3(16.9)
|
53.6 (5.8)
|
51.0 (3.9)
|
0.000 b
|
119.6 (15.1)
|
57.8 (7.6)
|
45.3 (3.4)
|
0.000 b
|
67.5 (105.9)
|
188 (60.1)
|
0.181c
|
|
|
Median
|
39
|
81
|
29
|
36
|
|
84
|
39
|
28
|
|
35
|
41
|
|
|
|
Min - Max
|
0 - 1087
|
0 - 1087
|
1 - 457
|
1 - 404
|
|
0 - 1087
|
0 - 457
|
0 - 404
|
|
0 – 1087
|
0 - 384
|
|
|
|
< 60
|
266 (66.0)
|
32 (41.6)
|
103 (74.1)
|
131 (70.1)
|
0.000 a
|
33 (37.9)
|
53 (70.7)
|
180 (74.7)
|
0.000 a
|
142 (66.0)
|
124 (66.0)
|
0.804a
|
|
|
61-90
|
58 (14.4)
|
11 (14.3)
|
19 (13.7)
|
28 (15.0)
|
|
14 (16.1)
|
11 (14.7)
|
33 (13.7)
|
|
29 (13.5)
|
29 (15.4)
|
|
|
|
> 90
|
79 (19.6)
|
34 (44.2)
|
17 (12.2)
|
28 (15.0)
|
|
40 (46.0)
|
11 (14.7)
|
28 (11.6)
|
|
44 (20.5)
|
35 (18.6)
|
|
|
*Totals may vary due to
missing values; **Surgery only or combined with radiation (with or without
chemotherapy); ***Radiation with or without brachytherapy. aChi-square
test; bKruskal-Wallis test; cMann-Whitney test.
Table 3. Prevalence of delay in
treatment initiation* and prevalence ratio according to stage at diagnosis in a
cohort of women diagnosed with cervical cancer and treated in Rio Branco, Acre,
between 2012 and 2017
|
Characteristics
|
All stages
|
|
IA1 - IIA2
|
IIB – IIIA
|
IIIB - IVA
|
|
Prevalence
|
PR (95%CI)
|
p-value
|
Prevalence
|
PR (95%CI)
|
Prevalence
|
PR (95%CI)
|
Prevalence
|
PR (95%CI)
|
|
Age at
admission
|
(%)
|
|
|
(%)
|
|
(%)
|
|
(%)
|
|
|
< 40
|
21.2
|
1
|
|
47.8
|
1
|
20.7
|
1
|
11.5
|
1
|
|
> 40
|
39.0
|
1.83 (1.25-2.69)
|
0.002
|
67.2
|
1.40 (0.72-2.72)
|
34.8
|
1.68 (0.65-4.29)
|
30.0
|
2.61 (1.19-5.74)
|
|
Residence
|
|
|
|
|
|
|
|
|
|
|
Rio Branco
|
36.4
|
1
|
|
53.5
|
1
|
34.3
|
1
|
30.2
|
1
|
|
Others
|
32.0
|
0.87 (0.66-1.15)
|
0.347
|
70.5
|
1.31 (0.93-1.84)
|
25.0
|
0.72 (0.36-1.47)
|
21.5
|
0.71 (0.46-1.09)
|
|
Skin color
|
|
|
|
|
|
|
|
|
|
|
Brown
|
33.8
|
1
|
|
63.3
|
1
|
25.0
|
1
|
25.2
|
1
|
|
Not Brown
|
38.7
|
1.14 (0.80-1.63)
|
0.459
|
63.6
|
1.00 (0.61-1.63)
|
60.0
|
2.40 (1.21-4.73)
|
26.8
|
1.06 (0.59-1.89)
|
|
Education
|
|
|
|
|
|
|
|
|
|
|
High school and
college
|
31.1
|
1
|
|
54.2
|
1
|
15.4
|
1
|
21.6
|
1
|
|
Illiterate and
elementary school
|
36.3
|
1.16 (0.79-1.70)
|
0.423
|
64.4
|
1.19 (0.77-1.82)
|
36.6
|
2.37 (0.62-9.05)
|
27.8
|
1.28 (0.66-2.50)
|
|
Comorbidities
|
|
|
|
|
|
|
|
|
|
|
No
|
35.4
|
1
|
|
68.4
|
1
|
32.5
|
1
|
26.7
|
1
|
|
Yes
|
36.6
|
1.03 (0.77-1.36)
|
0.825
|
59.4
|
0.86 (0.60-1.24)
|
33.3
|
1.02 (0.51-2.05)
|
29.1
|
1.08 (0.70-1.68)
|
|
Smoking
history
|
|
|
|
|
|
|
|
|
|
|
No
|
26.5
|
1
|
|
52.0
|
1
|
25.9
|
1
|
18.8
|
1
|
|
Yes
|
37.7
|
1.42 (1.02-1.98)
|
0.037
|
63.6
|
1.22 (0.79-1.89)
|
31.7
|
1.22 (0.56-2.66)
|
31.1
|
1.65 (0.98-2.79)
|
|
Alcohol
history
|
|
|
|
|
|
|
|
|
|
|
No
|
27.5
|
1
|
|
58.6
|
1
|
26.5
|
1
|
20.2
|
1
|
|
Yes
|
41.5
|
1.51 (1.12-2.03)
|
0.007
|
60.5
|
1.03 (0.69-1.53)
|
34.4
|
1.29 (0.62-2.71)
|
36.0
|
1.78 (1.13-2.80)
|
|
Histological type
|
|
|
|
|
|
|
|
|
|
|
Squamous cell
carcinoma
|
33.9
|
1
|
|
63.3
|
1
|
29.6
|
1
|
25.2
|
1
|
|
Adenocarcinoma
|
34.8
|
1.02 (0.57-1.82)
|
0.934
|
50.0
|
0.79 (0.38-1.61)
|
25.0
|
0.84 (0.14-4.79)
|
27.3
|
1.08 (0.40-2.91)
|
|
Time to first
specialist consultation
|
|
|
|
|
|
|
|
|
|
|
< 30 days
|
20.3
|
1
|
|
55.6
|
1
|
16.7
|
1
|
10.4
|
1
|
|
> 30 days
|
65.6
|
3.23 (2.48-4.21)
|
0.000
|
72.7
|
1.30 (0.95-1.79)
|
61.9
|
3.71 (1.87-7.36)
|
63.2
|
6.07 (3.78-9.75)
|
|
1st surgical
treatment
|
|
|
|
|
|
|
|
|
|
|
No
|
28.2
|
1
|
|
38.5
|
1
|
31.0
|
1
|
26.2
|
1
|
|
Yes
|
58.4
|
2.07 (1.60-2.67)
|
0.000
|
72.1
|
1.87 (1.12-3.12)
|
-
|
-
|
8.3
|
0.31 (0.04-2.10)
|
|
1st treatment
radiotherapy
|
|
|
|
|
|
|
|
|
|
|
No
|
57.7
|
1
|
|
72.1
|
1
|
-
|
1
|
8.3
|
1
|
|
Yes
|
28.3
|
0.49 (0.37-0.63)
|
0.000
|
38.5
|
0.53 (0.32-0.88)
|
31.4
|
-
|
26.2
|
3.14 (0.47-20.79)
|
* <60 days or
>60 days.
In Table 4, delays were
independently associated with age >40 years (PR 1.75; 95%CI 1.26-2.42), time
>30 days to first specialist consultation (PR 2.94; 95%CI 2.27-3.81), and
stage IIIB-IVA (PR 0.51; 95%CI 0.35-0.76).
Table 4. Crude and adjusted prevalence
ratios (PR) for delay in treatment initiation* in a cohort of women diagnosed
with cervical cancer in Rio Branco, Acre, between 2012 and 2017
|
|
Crude PR (95% CI)
|
Adjusted PRa (95% CI)
|
|
Age at admission
|
|
|
|
<40
|
1
|
1
|
|
>40
|
1.83 (1.25-2.69)
|
1.75 (1.26-2.42)
|
|
Time to first specialist consultation
|
|
|
|
< 30 days
|
1
|
1
|
|
> 30 days
|
3.23 (2.48-4.21)
|
2.94 (2.27-3.81)
|
|
Treatment protocol
|
|
|
|
Surgery**
|
1
|
1
|
|
Radiation Only or Chemoradiation
|
0.48 (0.37-0.62)
|
0.80 (0.54-1.19)
|
|
Stage
|
|
|
|
IA1 – IIA2
|
1
|
1
|
|
IIB – IIIA
|
0.47 (0.32-0.69)
|
0.64 (0.40-1.03)
|
|
IIIB – IVA
|
0.40 (0.31-0.53)
|
0.51 (0.35-0.76)
|
*<60 days or
>60 days; **Surgery only or combined with radiation (with or without
chemotherapy). aAdjusted by all other variables in the model.
DISCUSSION
In Rio Branco, Acre, women
with cervical cancer stages IA1 to IVA experienced a median time of 39 days
between diagnosis and treatment initiation, with a 34% prevalence of delays
(>60 days). These results comply with the 60-day range (law 12,732/2012) to
initiate treatment. Factors positively associated with treatment delays
included age >40 years and waiting >30 days for specialist consultation.
Conversely, advanced stages IIIB-IVA were factors negatively associated with
delays. The waiting times for treatment initiation observed in this study
suggest that the oncology service in Rio Branco is more organized and better at
complying with the legal deadlines for oncological treatment compared to other
regions in Brazil. For instance, in Rio de Janeiro, the median waiting time is
114 days, with a delay prevalence (>60 days) of 92.8% for the first
treatment28. In another study conducted in
Paraná, the prevalence of delay was 62.2%38. This result is
surprising given the high incidence and mortality indicators in the North
compared to the Southeast and South regions, respectively.
During the period studied,
there were reports of defective radiotherapy equipment requiring skilled
servicing. In
such circumstances, patients were referred to other reference centers of the
SUS network to continue treatment via OHT and returned to continue therapy and
follow-up. Despite these inconveniences and the bureaucracy involved in the
process, radiotherapy treatment proved to be a protective factor against delays
in treatment initiation, with a crude prevalence ratio of 0.49 (95%IC
0.37-0.63). Although it did not reach statistical significance in the adjusted
model, it remained a protective factor.
However, the scarcity of skilled
equipment servicing in Acre often negatively influences this process. Patients
wouldn't need to be referred if timely preventive and corrective equipment maintenance
were available, in addition to reducing costs for the institution. Thus, women
referred to OHT had a median of 41 days to treatment initiation, which was
longer than the 35 days for those not referred, but still within the
recommended 60-day timeframe.
Other results of this
study, as the high percentage of advanced-stage diagnoses and the effect of
surgery on treatment initiation delay, seem to corroborate findings from
studies conducted in other regions of the country. In Rio Branco, the frequency
of advanced staging at diagnosis (IIB-IVA) was considered high (78.4%) compared
to Belém, PA (30.7%) in the North39, Rio de Janeiro (67.7%)
in the Southeast28, Porto Alegre (77.3%) and Paraná (73.9) in the South
regions26,38. In this context, most women with advanced-stage
cervical cancer (94.93%) received treatment with exclusive radiation or
chemoradiation, with very low median waiting times of 29 and 36 days,
respectively. In contrast, the median waiting time for surgical treatment was
81 days. This extended waiting time aligns with findings from a study conducted
in Rio de Janeiro, where women who underwent surgery had a median time of 115
days between diagnosis and treatment initiation28. Another study conducted
in Paraná shows that 71% of women submitted to surgery as their first treatment
experienced a delay of more than 60 days26.
Although the mean prevalence
of delays in treatment initiation was considered low in Rio Branco, these
findings may raise concerns. The survival rate of women treated at early stages
is higher than that of women treated at locally advanced and advanced stages17,19,40,41. Delays in treatment initiation for early-stage patients who
usually undergo surgery (62.1%) could negatively impact the survival of this
subgroup in Rio Branco. However, this result is similar to those observed in
cities in more developed regions, as Rio de Janeiro (Southeast) and Paraná
(South), where the prevalence of delays in treatment initiation among women
undergoing surgery ranged from 79.4% to 71%, respectively26,28.
These findings contrast
with those of a population-based study32 which reported a higher
prevalence of treatment delays in the North region, with over 70% of women
initiating treatment in 60+ days after diagnosis. This high percentage remained
stable from 2013 to 2018 and only declined to around 60% in 2020. Although that
study provides important population-level insights, the estimates were based on
aggregated data and might be overestimated due to repeated counts of procedures
rather than individual patients. Moreover, all Northern states were grouped in
the analysis, disregarding structural and organizational differences in health
service provision across the region. By comparison, the present study employed
primary data collection from medical records and histopathological reports,
allowing more accurate and individualized estimates of treatment delays in
Acre. These findings, based on patient-level trajectories, portray a more
accurate picture of the situation in Rio Branco and underscore the value of
localized studies for regional health planning.
In this context, compared
to surgical treatment, it was observed that in Rio Branco, treatments with
exclusive radiation and chemoradiation were protective factors against delays
in treatment initiation, as opposed to a study conducted in Rio de Janeiro,
where surgical treatment was shown to be a protective factor against delays in
treatment initiation28. This result can be
explained by the fact that, in the state of Acre, a single reference center
accredited by SUS (Unacon) located in the capital, performs oncological
treatment of cervical neoplasms using external radiation (teletherapy),
internal radiation (brachytherapy), and chemotherapy.
In contrast, surgical
treatments are performed outside Unacon based on referral and counter-referral
systems. After surgical treatment, patients return to Unacon for post-treatment
follow-up. In the study conducted in Rio de Janeiro, surgical treatments were
performed at the institution where the patient was enrolled, while radiotherapy
treatments rely on the regulatory system for referral. Consequently, the likelihood
of delays in chemoradiation treatment initiation for patients in Rio de Janeiro
was 1.84 times higher compared to those who underwent surgical treatment28.
Therefore, Brazilian
bureaucratic processes for regulating referrals, planning surgeries,
availability of vacancies, and waiting lists for surgical procedures could
partially explain the delays in surgical treatment initiation observed in Rio
Branco. To reduce the waiting time to start treatment, oncology care centers
need a structure that allows the performance of all treatments for patients
with cervical cancer, avoiding the referral through regulatory systems. Additionally,
evidence suggests a subjective influence of health professionals, who may
prioritize patients with more severe signs and symptoms, as intense bleeding
and pain, instead of those in the early stages of the disease10,12.
A delay of more than 30
days between diagnosis and the first consultation with a specialist was
independently associated with delayed treatment initiation. The prevalence
ratio for this delay was 3.23 (95% CI 2.48-4.21), compared to those who had
their first specialist consultation within 30 days. This prolonged time between
diagnosis and the first specialized care reflects the quality of the health
services offered and provided in the health unit13-5,42. On the
other hand, delays may result from structural problems, as the geographical
distance of the health unit where treatment is offered and the availability of
transportation. Non-structural problems, as individual factors of the patient
and the doctor, may also contribute8,16,42,43.
The variable “time to
first specialist consultation” was included to explore its association with
delayed treatment initiation, despite sharing the same reference point
(diagnosis date) as the outcome. While this temporal overlap limits the
independence between the variables, the analysis was conducted cautiously and
without the intention of establishing causality. This choice aimed to highlight
potential delays in different stages of the care pathway and to identify
systemic bottlenecks that may contribute to treatment delays.
Thus, in the present
study, women aged 40 or older had a prevalence ratio of 1.83 for delays in
treatment initiation, compared to those under 40, which indicates an 83% higher
likelihood of experiencing delays. It is also worth highlighting that 62.5% of
this cohort had a history of smoking, and 46.6% had a history of alcohol use.
In crude analyses, both factors were associated with delays in treatment
initiation, with prevalence ratios of 1.42 and 1.51, respectively.
To the best existing
knowledge, this groundbreaking study is the first in the Western Brazilian
Amazon to describe the waiting time in the therapeutic trajectory for women
diagnosed with cervical cancer at stages IA1 and IVA. It stands out due to its
meticulous data collection from all medical records of women diagnosed with
cervical cancer and treated at hospitals in Rio Branco, Acre. The diagnosis and
treatment data were confirmed through histopathological reports and medical
records, ensuring the utmost accuracy and reliability of the findings.
In addition, the study
evaluated the role of the OHT program in delays in treatment initiation, which
is a very relevant factor for a state as isolated as Acre, further to the low
percentage of patient and data losses, reducing the risk of selection bias and
increasing its validity.
Finally, considering that
the Unacon is the only oncology treatment center of SUS in the entire state of
Acre that offers radiotherapy treatment and that “Hospital Santa Juliana”
is the private reference hospital for gynecological surgeries in the city of
Rio Branco, the population of women with cervical cancer in this study could
approximately represent the entire state of Acre.
The study limitations are
inherent to retrospective studies based on data collection from medical records.
Among these, the lack of information about the reality of women at the time of
treatment may have contributed to delays, as socioeconomic conditions, lack of
knowledge of the clinical condition, or geographical limitations for health
monitoring. Another limitation was the lack of information before diagnosis, as
the screening history of these women, which is generally done in primary care.
Even with the computerization of data in health institutions, including SUS,
the systems are not interconnected, preventing access to basic information and
previous follow-up of patients.
CONCLUSION
When evaluating the
population, the time between diagnosis and treatment initiation showed a median
of 39 days, which is within the law-mandated 60-day in Brazil. However, when
stratifying these results by treatment protocols performed and by staging,
surgical protocols and initial staging showed medians of 81 and 84 days
respectively, outside the recommended period.
Despite the consensus
regarding access and treatments in the North region, and the high incidence and
mortality rates, Acre surprisingly presents good results of delays in cervical
cancer treatment initiation when compared with studies carried out in the South
and Southeast regions.
Older ages and the time
between diagnosis and the first consultation with a specialist were positively
associated with the likelihood of delay, while advanced stages were inversely
associated with delay in treatment initiation. The implementation of
interconnected information systems would facilitate the construction of the
therapeutic pathway from screening to treatment for these women, contributing
to wider analysis and new studies. Prospective studies should be conducted to
identify and monitor delays related to patient’s factors and investigate the
effects of delays on treatment response and survival.
ACKNOWLEDGMENTS
To the entire team of “Programa
de Pós-Graduação em Saúde Coletiva” of “Universidade Federal do Acre (UFAC)”,
the “Secretaria Estadual de Saúde do Acre - SESACRE” and “Hospital
Santa Juliana”, Acre, for all their support in developing the study.
CONTRIBUTIONS
Ilce Ferreira da Silva
contributed to the conceptualization and design of the study, supervision,
review and writing. Liz Rodrigues de Souza contributed to the analysis, data
curation, supervision and writing. Maria Fernanda de Sousa Oliveira Borges contributed
to data curation, supervision and writing. They approved the final version for
publication.
DECLARATION OF CONFLICT OF
INTERESTS
There is no conflict of
interests to declare.
DATA AVAILABILITY STATEMENT
Given ethical and confidentiality
issues, data can be requested to the corresponding author with reasonable
justification.
FUNDING SOURCES
Partially funded by “Coordenação
de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES)” – Code
001.
REFERENCES
1. Ferlay J, Ervik
M, Lam F, et al. Global Cancer Observatory: cancer today [Internet]. Lyon:
International Agency for Research on Cancer; 2020 [acesso 2024 jun13].
Disponível em: https://gco.iarc.who.int/today/
2. Silva GA, Jardim BC,
Ferreira VM, et al. Cancer mortality in the capitals and in the interior of Brazil: a
four-decade analysis. Rev Saúde Pública. 2020;54:126. doi: https://doi.org/10.11606/s1518-8787.2020054002255
3. Jardim BC,
Junger WL, Daumas RP, et al. Estimativa de incidência de câncer no Brasil e regiões em 2018: aspectos
metodológicos. Cad Saúde Pública. 2024;40(6):e00131623. doi: https://doi.org/10.1590/0102-311XPT131623
4. Incidências do BasePop
[Internet]. Rio de Janeiro: INCA; 2023. [acesso 2024 jun 13]. Disponível em: https://www.inca.gov.br/BasePopIncidencias/Home.action
5. Nakashima JDP, Koifman RJ, Koifman S.
Cancer incidence in the Western Amazon: population-based estimates in Rio
Branco, Acre State, Brazil, 2007-2009. Cad Saúde Pública. 2012;28(11):2125-32.
6. International
Agency for Research on Cancer. Cervical cancer screening [Internet]. Lyon:
IARC; 2022. [acesso 2024
jun 13]. Disponível em: https://publications.iarc.fr/_publications/media/download/5768/2ede2d8620766fdfd85bf922f28c91ec123274f1.pdf
7. Prendiville W,
Sankaranarayanan R. Colposcopy and treatment of cervical precancer [Internet].
Lyon: International Agency for Research on Cancer; 2017. [acesso 2024 jun 13]. Disponível
em: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Technical-Publications/Colposcopy-And-Treatment-Of-Cervical-Precancer-2017
8. Vaccarella S,
organizador. Reducing social inequalities in cancer: evidence and priorities
for research. Lyon: International Agency for Research on Cancer; 2019. (IARC
Scientific Publications; 168).
9. World Health
Organization. WHO guideline for screening and treatment of cervical pre-cancer
lesions for cervical cancer prevention. 2. ed. Geneva: World Health
Organization; 2021.
10. Bhatla N, Aoki D,
Sharma DN, et al. Cancer of the cervix uteri: 2021 update. Int J Gynaecol
Obstet. 2021;155(S1):28-44. doi: https://doi.org/10.1002/ijgo.13865
11. Abu-Rustum NR,
Yashar CM, Arend R, NCCN Guidelines® Insights: cervical cancer, Version 1.2024.
J Natl Compr Canc Netw. 2023;21(12):1224-33. doi: https://doi.org/10.6004/jnccn.2023.0062
12. Marth C, Landoni
F, Mahner S, et al. Cervical cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 2017;28(sup4):iv72-83. doi: https://doi.org/10.1093/annonc/mdx220
13. Hansen RP,
Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment
of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health
Serv Res. 2011;11(1):284. doi: https://doi.org/10.1186/1472-6963-11-284
14. Robinson KM,
Ottesen B, Christensen KB, et al. Diagnostic delay experienced among
gynecological cancer patients: a nationwide survey in Denmark. Acta Obstet Gynecol Scand.
2009;88(6):685-92. doi: https://doi.org/10.1080/00016340902971482
15. Santos TTDM, Andrade LSDS,
Oliveira MECD, et al. Availability of diagnostic services and their impact on
patient flow in two brazilian referral centres of breast cancer treatment. Asian
Pac J Cancer Prev. 2020;21(2):317-24. doi: https://doi.org/10.31557/apjcp.2020.21.2.317
16. Carvalho PGD, O´Dwer G,
Rodrigues NCP. Trajetórias assistenciais de mulheres entre diagnóstico e início
de tratamento do câncer de colo uterino. Saúde debate. 2018;42(118):687-701. doi:
https://doi.org/10.1590/0103-1104201811812
17. Brookfield KF,
Cheung MC, Lucci J, et al. Disparities in survival among women with invasive
cervical cancer: a problem of access to care. Cancer. 2009;115(1):166-78.
18. Perri T, Issakov
G, Ben-Baruch G, et al. Effect of treatment delay on survival in patients with
cervical cancer: a historical cohort study. Int J Gynecol Cancer. 2014;24(7):1326-32.
doi: https://doi.org/10.1097/igc.0000000000000211
19. Silva IF, Silva IF, Saraceni V, et al.
Delays in
treatment initiation and conclusion in women with stage IA to IIIB cervical
cancer: a survival study in a hospital-based cohort from a developing country. Cancer
Epidemiol. 2023;86:102450. doi: https://doi.org/10.1016/j.canep.2023.102450
20. Chen SW, Liang
JA, Yang SN, et al. The adverse effect of treatment prolongation in cervical
cancer byhigh-dose-rate intracavitary brachytherapy. Radiother Oncol. 2003;67(1):69-76. doi:
https://doi.org/10.1016/s0167-8140(02)00439-5
21. Nascimento MID, Azevedo e
Silva G. Efeito do tempo de espera para radioterapia na sobrevida geral em
cinco anos de mulheres com câncer do colo do útero, 1995-2010. Cad Saúde
Pública. 2015;31(11):2437-48. doi: https://doi.org/10.1590/0102-311X00004015
22. McLaughlin JM, Anderson RT, Ferketich
AK, et al. Effect on survival of longer intervals between confirmed diagnosis
and treatment initiation among low-income women with breast cancer. J Clin
Oncol. 2012;30(36):4493-500. doi: https://doi.org/10.1200/jco.2012.39.7695
23. Weller D, Vedsted
P, Rubin G, et al. The Aarhus statement: improving design and reporting of
studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262-7. doi: https://doi.org/10.1038/bjc.2012.68
24. Chen CP, Kung PT,
Wang YH, et al. Effect of time interval from diagnosis to treatment for
cervical cancer on survival: a nationwide cohort study. PLoS One. 2019;14(9):e0221946. doi: https://doi.org/10.1371/journal.pone.0221946
25. Presidência da República (BR).
Lei no 12.732 de 22 de novembro de 2012. Dispõe sobre o primeiro tratamento de
paciente com neoplasia maligna comprovada e estabelece prazo para seu início.
Diário Oficial da União [Internet], Brasília, DF. 2012 nov 23 [acesso 2024 jun
13]; Edição 226; Seção 1:1. Disponível em: https://pesquisa.in.gov.br/imprensa/jsp/visualiza/index.jsp?jornal=1&pagina=1&data=23/11/2012&totalArquivos=240
26. Assenço KC, Kluthcovsky
ACGC, Mansani FP. Atraso no diagnóstico e tratamento de pacientes com câncer de
colo de útero atendidas pelo Sistema Único de Saúde em um centro de referência
do Sul do Brasil. Mundo Saúde. 2017;41(4):692-702.
27. Nakagawa JT, Espinosa MM,
Barbieri M, et al. Carcinoma do colo do útero: taxa de sobrevida e fatores
prognósticos em mulheres no Estado de Mato Grosso. Acta Paulista de Enfermagem.
2011;24(5):631-7. doi: https://doi.org/10.1590/S0103-21002011000500006
28. Silva IF, Silva IF, Koifman RJ. Cervical
cancer treatment delays and associated factors in a cohort of women from a
developing country. J
Glob Oncol. 2019;(5):1-11. doi: https://doi.org/10.1200/jgo.18.00199
29. Silva DS, Pinto MC,
Figueiredo MAA. Factors associated with delay in specialized treatment after diagnosis of
cervical cancer in Bahia State, Brazil. Cad Saúde Pública.
2022;38(5):e00022421. doi: https://doi.org/10.1590/0102-311xpt022421
30. Jensen OM,
International Agency for Research on Cancer, World Health Organization, et al.,
organizadores. Cancer registration: principles and methods. Lyon: International
Agency for Research on Cancer; New York: Distributed in the USA by Oxford
University Press; 1991. 288 p. (IARC scientific publications).
31. Girianelli VR,
Gamarra CJ, Azevedo e Silva G. Disparities in cervical and breast cancer
mortality in Brazil. Rev
Saúde Pública. 2014;48(3):459-67. doi: https://doi.org/10.1590/S0034-8910.2014048005214
32. Silva GA, Alcantara LLM,
Tomazelli JG, et al. Avaliação das ações de controle do câncer de colo do útero
no Brasil e regiões a partir dos dados registrados no Sistema Único de Saúde.
Cad Saúde Pública. 2022;38(7):e00041722. doi: https://doi.org/10.1590/0102-311XPT041722
33. Organização Mundial da
Saúde. CID-10: Classificação Estatística Internacional de Doenças e problemas
relacionados à saúde. São Paulo: Edusp; 2008.
34. Coutinho LMS, Scazufca M,
Menezes PR. Methods for estimating prevalence ratios in cross-sectional
studies. Rev Saúde Pública. 2008;42:992-8.
35. Barros AJ, Hirakata VN. Alternatives
for logistic regression in cross-sectional studies: an empirical comparison of
models that directly estimate the prevalence ratio. BMC Med Res Methodol.
2003;3(1):21. doi: https://doi.org/10.1186/1471-2288-3-21
36. SPSS®:
Statistical Package for Social Science (SPSS) [Internet]. Versão 20.0. [Nova
York]. International Business Machines Corporation. [acesso 2023 mar 9].
Disponível em: https://www.ibm.com/br-pt/spss?utm_content=SRCWW&p1=Search&p4=43700077515785492&p5=p&gclid=CjwKCAjwgZCoBhBnEiwAz35Rwiltb7s14pOSLocnooMOQh9qAL59IHVc9WP4ixhNTVMjenRp3-aEgxoCubsQAvD_BwE&gclsrc=aw.ds
37. Conselho Nacional de Saúde
(BR). Resolução n° 466, de 12 de dezembro de 2012. Aprova as diretrizes e
normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial
da União, Brasília, DF. 2013 jun 13; Seção I:59.
38. Alves RJV, Watte G, Garcez
ADS, et al. Assessment
of survival in patients with cervical cancer in a hospital based cohort in
Southern Brazil. Braz J Oncolog. 2017;13(46). doi: https://www.doi.org/10.26790/BJO20171346A74
39. Carneiro SR, Fagundes MDA, Rosário
PDJO, et al. Five-year
survival and associated factors in women treated for cervical cancer at a
reference hospital in the Brazilian Amazon. PLoS One. 2017;12(11):e0187579. doi:
https://doi.org/10.1371/journal.pone.0187579
40. Chen CP, Kung PT,
Wang YH, et al. Effect of time interval from diagnosis to treatment for
cervical cancer on survival: a nationwide cohort study. PLoS One
2019;14(9):e0221946. doi: https://doi.org/10.1371/journal.pone.0221946
41. Wassie M, Argaw
Z, Tsige Y, et al. Survival status and associated factors of death among
cervical cancer patients attending at Tikur Anbesa Specialized Hospital, Addis
Ababa, Ethiopia: a retrospective cohort study. BMC Cancer. 2019;19(1):1221. doi:
https://doi.org/10.1186/s12885-019-6447-x
42. Ambroggi M,
Biasini C, Del Giovane C, et al. Distance as a barrier to cancer diagnosis and
treatment: review of the literature. Oncologist. 2015;20(12):1378-85. doi: https://doi.org/10.1634/theoncologist.2015-0110
43. Davis TC, Williams MV, Marin
E, et al. Health literacy and cancer communication. CA Cancer J Clin. 2002;52(3):134-49. doi: https://doi.org/10.3322/canjclin.52.3.134
Recebido em 7/5/2025
Aprovado em 15/7/2025
Scientific-editor: Anke Bergmann. Orcid iD: https://orcid.org/0000-0002-1972-8777

Este é um artigo publicado em acesso aberto (Open Access) sob a
licença Creative Commons Attribution, que permite
uso, distribuição e reprodução em qualquer meio, sem restrições, desde que o
trabalho original seja corretamente citado.
